Sodium salicylate is the sodium salt of salicylic acid.
The name of this compound gives us information about its structure and its components:
- Sodium indicates a sodium ion in the compound. Sodium ions are positively charged and help increase the solubility of the compound in water.
- Salicylate comes from "salicylic acid", indicating that the compound is derived from a type of phenolic acid known for its ability to relieve pain and reduce inflammation.
The synthesis process takes place in several stages:
- Sodium salicylate can be synthesized by the reaction of salicylic acid with sodium hydroxide, resulting in the formation of sodium salicylate and water.
- Another method of synthesis involves the reaction of sodium phenolate (the sodium salt of phenol) with carbon dioxide, forming sodium salicylate. This reaction is carried out at high pressure and temperature.
- Sodium salicylate can also be synthesized through the metathesis reaction of docusate sodium or sodium NSAID salts with phenothiazine hydrochloride salts. This reaction causes the formation of liquid co-crystals with varying degrees of ionization.
- It can also be synthesized from the reaction of a metathesis product of 1-methyl-3-(3-trimethoxysilylpropylimidazolium) chloride [MTMSPI][Cl] with sodium salicylate through an ionically exchanged reaction. This reaction causes the formation of a solid-supported ionic liquid [MTMSPI][Sal].
It occurs as a fine, white powder or in tiny crystals. Soluble in water, glycerine and sparingly soluble in alcohol, insoluble in ether, chloroform, benzene and other organic solvents.

What it is used for and where
The first non-steroidal anti-inflammatory drugs (NSAIDs) were salicylates for their anti-inflammatory and analgesic properties. The best-known applications of salicylates are aspirin (acetylsalicylic acid) and sodium salicylate, both of which are inexpensive and readily available.
Medicine
Analgesic, antipyretic, non-steroidal anti-inflammatory. Also used as a substitute for aspirin. Against keratosis, dandruff and others. In skin infections caused by fungus.
As a reagent to measure free acid in gastric juices. as an analytical reagent.
In spot microanalysis to determine uranium dioxide and organic synthesis
Cosmetics
It is a restricted ingredient as V/3 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Maximum concentration in ready for use preparation: Salicylic acid 0,5 % (acid). Salicylic acid salts: 0,5 % (acid).
Denaturant. It makes cosmetics unpalatable. It is sometimes added to cosmetics containing ethyl alcohol to make it unsuitable for ingestion. The ionic or polar molecules of this ingredient included in formulations that interact with protein groups, modulate the properties of the solution to suit specific needs.
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Studies
Aspirin is preventive against stroke not only because of its antithrombotic properties but also because of other direct effects. The aim of this study was to elucidate its direct neuroprotective effects. The findings of this study show a new mechanism for the neuroprotective effects of aspirin, which occurs at concentrations in the antithrombotic-analgesic range, useful in the management of patients at high risk of ischaemic events (1).
Tinnitus is thought to be caused by damage to the auditory and non-auditory systems due to exposure to loud noises, ageing or other aetiologies. Sodium salicylate (NaSal) was used to create an animal model of rats with tinnitus, assessed with prepulsive inhibition behaviour. The results showed that the acquisition and recovery of spatial memory was impaired by treatment with NaSal (2).
Sodium salicylate showed inhibitory activity of quorum sensing, a cell density-dependent signalling system that controls the production of many virulence factors and biofilm formation in Pseudomonas aeruginosa and is therefore to be considered as an adjuvant in delayed healing of chronic wounds (3).
The most relevant studies on this chemical compound have been selected with a summary of their contents:
Sodium salicylate studies
Optimal typical characteristics of the commercial product Sodium salicylate
| Appearance | Colorless crystal or crystal slice powder |
| Boiling Point | 336.3ºC at 760mmHg |
| Melting Point | >300 °C(lit.) |
Flash Point
| 144.5ºC |
| PSA | 60.36000 |
Index of Refraction
| 1.435 |
| Acidity | 2.0ml of 0.01M NaOH |
Heavy metal
| ≤20PPm |
Loss on drying
| ≤0.5% |
| Sulphate | ≤600PPm |
| Safety |  |
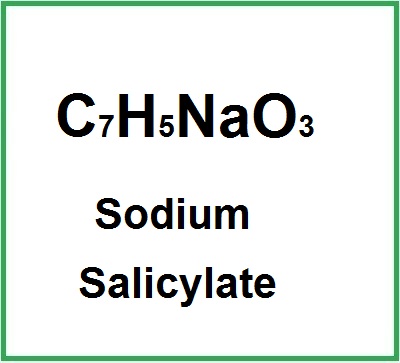
- Molecular Formula : C7H5NaO3
- Linear Formula HOC6H4COONa
- Molecular Weight : 160.104 g/mol
- Exact mass 160.013641
- CAS : 54-21-7 90218-97-6
- UNII WIQ1H85SYP
- EC Number: 200-198-0
- DSSTox Substance ID: DTXSID5021708
- MDL number MFCD00002440
- PubChem Substance ID 24899540
- Beilstein 3732792
- InChI=1S/C7H6O3.Na/c8-6-4-2-1-3-5(6)7(9)10;/h1-4,8H,(H,9,10);/q;+1/p-1
- InChl Key ABBQHOQBGMUPJH-UHFFFAOYSA-M
- SMILES C1=CC=C(C(=C1)C(=O)[O-])O.[Na+]
- IUPAC sodium;2-hydroxybenzoate
- ChEBI 9180
- NACRES NA.25
- ICSC
- NSC 202167
- RTECS
- UN
- NCI C834
Synonyms:
- Sodium 2-hydroxybenzoate
- Monosodium 2-hydroxybenzoate
- Salicylic acid sodium salt
- o-Hydroxybenzoic acid, sodium salt
- 2-Hydroxybenzoic acid monosodium salt
- sodium;2-hydroxybenzoate
- Enterosalicyl
- Enterosalil
- Entrosalyl
- Glutosalyl
- Kerasalicyl
References______________________________________________________________________
(1) De Cristóbal J, Cárdenas A, Lizasoain I, Leza JC, Fernández-Tomé P, Lorenzo P, Moro MA. Inhibition of glutamate release via recovery of ATP levels accounts for a neuroprotective effect of aspirin in rat cortical neurons exposed to oxygen-glucose deprivation. Stroke. 2002 Jan;33(1):261-7. doi: 10.1161/hs0102.101299.
(2) Niu H, Ding S, Li H, Wei J, Ren C, Wu X, Huma T, Zhang Q. Effect of Long-Term Sodium Salicylate Administration on Learning, Memory, and Neurogenesis in the Rat Hippocampus. Biomed Res Int. 2018 Apr 1;2018:7807426. doi: 10.1155/2018/7807426.
(3) Gerner E, Almqvist S, Werthén M, Trobos M. Sodium salicylate interferes with quorum-sensing-regulated virulence in chronic wound isolates of Pseudomonas aeruginosa in simulated wound fluid. J Med Microbiol. 2020 May;69(5):767-780. doi: 10.1099/jmm.0.001188.
![]() Sodium Salicylate
Sodium Salicylate