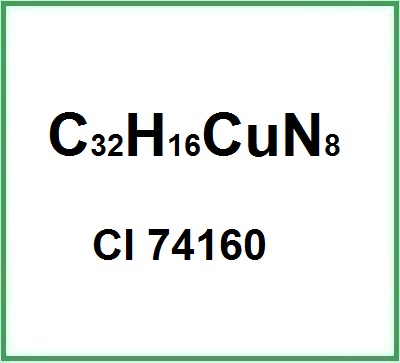
CI 74160 (Copper Phthalocyanine) is a chemical compound, it belongs to the sulfonated Metal phthalocyanines family with the name copper phthalocyanine-3,4′,4″,4″′-tetrasulfonic acid tetrasodium salt.
Chemical Composition and Structure
Copper Phthalocyanine is an organic compound in which a central copper atom is surrounded by a phthalocyanine ring. This structure gives the pigment its characteristic intense blue color. Its chemical formula is C32H16CuN8, comprising copper, carbon, hydrogen, and nitrogen, and its highly conjugated structure provides the pigment with remarkable stability and resistance to degradation from environmental factors.
Physical Properties
CI 74160 appears as a bright blue powder, insoluble in water but dispersible in oils and organic solvents. It is known for its excellent resistance to heat, sunlight, and chemicals, making it a stable and long-lasting pigment. It disperses well in various formulations without losing color intensity.

The name describes the structure of the molecule:
- Copper is the copper ion (Cu) a transition metal that can form complex ions with organic compounds.
- Phthalocyanine is a large aromatic macrocyclic compound consisting of four isoindole units linked by nitrogen atoms. The name is derived from 'phthalic acid' and 'cyanine', which are related to the structure of the compound. Phthalocyanines are often used as dyes and pigments due to their intense blue-green colour and stability.
Production Process
Copper Phthalocyanine is synthetically produced through the condensation of phthalonitriles with copper. The result is a stable, durable compound that can be further processed to achieve pigments of various particle sizes, depending on the requirements of the industry in which it is used.
- First Reaction . In the first phase, there is a reaction of phthalic anhydride with urea. This reaction produces phthalimide.
- Second Reaction. The phthalimide is reacted with ammonia, producing phthalonitrile.
- Polymerisation. Phthalonitrile is polymerised in the presence of a copper catalyst. Copper phthalocyanine is thus formed.
- Purification. The copper phthalocyanine is purified by a washing and filtration process.
What it is for and where
It is a fairly discussed chemical dye, used in
Cosmetics
It is a restricted ingredient as IV/105 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Sostanza o ingrediente a rischio: (29H,31H-Phthalocyaninato(2-)-N29,N30,N31,N32)copper
The CI 74160 also called Copper phthalocyanine and it is used in tattoo, cleaning products and various other applications to give a blue color.
Below are some interesting studies.
Tattoos
- Inserting inorganic or organic pigments into the dermis for the purpose of tattooing or permanent make-up represents an unbroken modern trend world-wide. At the same time, dramatically increasing numbers of tattooed people result in a similarly increasing demand for laser removal of tattoo pictures and designs that occasionally become subject of embarrassment and deepest regret later in life. In this regard the potential risk for pigment cleavage into toxic or carcinogenic fragments upon tattoo laser treatment or even just during the exposure of skin to regular light (e.g. while sunbathing) are increasingly recognized as a serious health-related issue. Applying dynamic headspace—gas chromatography with mass spectrometric detection (DHS—GC/MS) and comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GCxGC—ToF-MS), we identified 1,2-benzene dicarbonitrile, benzonitrile, benzene, and the poisonous gas hydrogen cyanide (HCN) as main fragmentation products emerging dose-dependently upon ruby laser irradiation of the popular blue pigment copper phthalocyanine in suspension. Skin cell viability was found to be significantly compromised at cyanide levels of ≥1 mM liberated during ruby laser irradiation of >1.5 mg/ml phthalocyanine blue. Further, for the first time we introduce pyrolysis-GC/MS as method suitable to simulate pigment fragmentation that may occur spontaneously or during laser removal of organic pigments in the living skin of tattooed people. According to the literature such regular tattoos hold up to 9 mg pigment/cm2 skin (1).
Warnings
Potential Acute Health Effects: Hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation (2).
Safety. Environmental pollution
- The textile dyeing industry is one of the main sectors contributing to environmental pollution, due to the generation of large amounts of wastewater loaded with dyes (ca. 2-50% of the initial amount of dyes used in the dye baths is lost), causing severe impacts on human health and the environment. In this context, an ecotoxicity testing battery was used to assess the acute toxicity and genotoxicity of the textile dyes Direct Black 38 (DB38; azo dye) and Reactive Blue 15 (RB15; copper phthalocyanine dye) on different trophic levels. Thus these dyes were tested using the following assays: Filter paper contact test with earthworms (Eisenia foetida); seed germination and root elongation toxicity test (Cucumis sativus, Lactuca sativa and Lycopersicon esculentum); acute immobilization test (Daphnia magna and Artemia salina); and the Comet assay with the rainbow trout gonad-2 cell fish line (RTG-2) and D. magna. Neither phytotoxicity nor significant effects on the survival of E. foetida were observed after exposure to DB38 and RB15. Both dyes were classified as relatively non-toxic to D. magna (LC50 > 100 mg/L), but DB38 was moderately toxic to A. salina with a LC50 of 20.7 mg/L. DB38 and RB15 induced significant effects on the DNA of D. magna but only DB38 caused direct (alkaline comet assay) and oxidative (hOGG1-modified alkaline comet assay) damage to RTG-2 cells in hormetic responses. Therefore, the present results emphasize that a test battery approach of bioassays representing multiple trophic levels is fundamental in predicting the toxicity of textile dyes, aside from providing the information required to define their safe levels for living organisms in the environment (3).
Commercial applications
Cosmetics. CI 74160 is used as a pigment in various cosmetic products like eyeshadows, nail polishes, and lipsticks to impart a blue hue.
Personal Care Products. It can be found in toothpastes, soaps, and other personal care products to color the product.
Plastic and Rubber Industry. Used as a dye for plastics and rubber.
Inks and Paints. Used as a pigment in inks and paints to give a deep blue color.
Food. In some regions, it might be used as a food dye, though its use in foods varies based on local regulations.
- Molecular Formula C32H16CuN8
- Molecular Weight 576.082
- CAS 147-14-8
- EC number 205-685-1
- UNII V5PUF4VLGY
Synonyms :
Pigment Blue 15
Copper phthalocyanine
Aqualine Blue
Fastogen Blue
Heliogen Blue
Cyanine Blue
References____________________________________________________________________
(1) Ines Schreiver, Christoph Hutzler, Peter Laux, Hans-Peter Berlien, Andreas Luch Formation of highly toxic hydrogen cyanide upon ruby laser irradiation of the tattoo pigment phthalocyanine blue Sci Rep. 2015
(2) http://www.sciencelab.com/msds.php?msdsId=9923554
(3) Oliveira GAR, Leme DM, de Lapuente J, Brito LB, Porredón C, Rodrigues LB, Brull N, Serret JT, Borràs M, Disner GR, Cestari MM, Oliveira DP. A test battery for assessing the ecotoxic effects of textile dyes. Chem Biol Interact. 2018 Aug 1;291:171-179. doi: 10.1016/j.cbi.2018.06.026. Epub 2018 Jun 22.
![]() CI 74160
CI 74160