![]() Decyl glucoside
Decyl glucoside
Rating : 6.1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Specific allergy (1)10 pts from FRanier
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Decyl glucoside, studies" about Decyl glucoside Review Consensus 7 by FRanier (9974 pt) | 2021-Dec-24 18:36 |
| Read the full Tiiip | (Send your comment) |
The Cosmetic Ingredient Review (CIR) Expert Panel assessed the safety of 19 alkyl glucosides used in cosmetics in 2013 and concluded that these ingredients are safe in current use practices and in their concentration when formulated to be non-irritant. These include Decyl glucoside (1).
However, despite being safe, Decyl glucoside has caused a number of cases of contact allergies (2).
With regard to soil pollution, it does not appear that Decyl glucoside significantly affects the environmental fate of glyphosate in soil at environmentally relevant concentrations (3).
References______________________________________________________________________
(1) Fiume MM, Heldreth B, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler D, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Safety assessment of decyl glucoside and other alkyl glucosides as used in cosmetics. Int J Toxicol. 2013 Sep-Oct;32(5 Suppl):22S-48S. doi: 10.1177/1091581813497764.
(2) Andrade P, Gonçalo M, Figueiredo A. Allergic contact dermatitis to decyl glucoside in Tinosorb M. Contact Dermatitis. 2010 Feb;62(2):119-20. doi: 10.1111/j.1600-0536.2009.01680.x.
Presley CL, Militello M, Barber C, Ladd R, Laughter M, Ferguson H, Dewey J, Pulsipher KJ, Rundle CW, Dunnick CA. The History of Surfactants and Review of Their Allergic and Irritant Properties. Dermatitis. 2021 Sep-Oct 01;32(5):289-297. doi: 10.1097/DER.0000000000000730.
Krehic M, Avenel-Audran M. Allergic contact dermatitis from decyl glucoside in an antiseptic lotion. Contact Dermatitis. 2009 Dec;61(6):349-50. doi: 10.1111/j.1600-0536.2009.01604.x.
Horn HM, Murray C, Aldridge RD. Contact allergy to decyl glucoside. Contact Dermatitis. 2005 Apr;52(4):227. doi: 10.1111/j.0105-1873.2005.0566b.x
(3) Carretta L, Cardinali A, Masin R, Zanin G, Cederlund H. Decyl glucoside surfactant Triton CG-110 does not significantly affect the environmental fate of glyphosate in the soil at environmentally relevant concentrations. J Hazard Mater. 2020 Apr 15;388:122111. doi: 10.1016/j.jhazmat.2020.122111.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Decyl glucoside Review Consensus 10 by FRanier (9974 pt) | 2024-Sep-20 17:24 |
| Read the full Tiiip | (Send your comment) |
Decyl Glucoside is a mild non-ionic surfactant derived from natural sources, specifically from corn glucose and fatty alcohols. It is widely used in cosmetic and personal care formulations for its cleansing and emulsifying properties, making it suitable for sensitive skin.
Chemical Composition and Structure
The chemical composition of Decyl Glucoside includes:
- Decyl Alcohol: A fatty alcohol that provides emulsification and surfactant properties.
- Glucose: A sugar that enhances the mildness and skin compatibility of the product.
Structurally, Decyl Glucoside is a glucoside, consisting of a decyl group linked to a glucose molecule, which contributes to its surfactant properties.
Physical Properties
- Appearance: Typically a clear to slightly yellow liquid.
- Solubility: Soluble in water; forms a stable solution.
- pH: Generally neutral to slightly acidic, around 6-7.
- Odor: Mild, characteristic of plant-derived ingredients.
- Stability: Stable under normal storage conditions; should be protected from extreme temperatures.
Production Process
- Synthesis: Decyl Glucoside is produced through the glycosylation of decyl alcohol with glucose, using mild reaction conditions to preserve the integrity of the components.
- Purification: The resulting product is purified to remove any unreacted materials and by-products.
- Formulation: The purified Decyl Glucoside is incorporated into various cosmetic products, often combined with other surfactants and ingredients to enhance performance.
Applications
- Medical: Limited use in formulations requiring gentle cleansing.
- Cosmetics: Commonly included in shampoos, body washes, facial cleansers, and other personal care products for its mild cleansing and foaming properties.

INCI Functions:
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Non-ionic, stabilising, PEG-free alkyl polyglucoside surfactant, designed for nanoparticle stabilisation (1). Stable foaming agent, rapidly biodegradable, low toxicity, low viscosity, low level of sodium salts. Has good alkali tolerance and electrolyte tolerance.
Used in cosmetics in liquid soaps and shampoos as a second surfactant with excellent caustic stability and solubility in highly concentrated salt, alkaline and surfactant solutions. Generally magnesium oxide and magnesium are present in amounts ranging from 100 ppm to 400 ppm.
Other uses:
- chemical industry
- oil extraction industry
- pesticide industry
Safety
The oestrogenicity of decyl glucoside was confirmed as a non-endocrine disrupting surfactant by its preparation method using zeolite catalysts. (2).
The Expert Group on Cosmetic Ingredient Control (CIR) evaluated the safety of 19 alkyl glucosides as used in cosmetics and concluded that these ingredients are safe in current use practices and concentrations when formulated to be non-irritating. (3).
For more information:
Typical optimal commercial product characteristics Caprylyl/Capryl Glucoside
| Appearance | Pale yellow liquid |
| Viscosity | 1000-2500 (mPa•s, 20℃) |
| Density (g/cm3), 25°C | 1.07-1.09 |
| Boiling Point | 476.5±45.0 °C at 760 mmHg |
| Flash Point | 242.0±28.7 °C |
C chain distribution C6 C8 C10 (base substance: Fatty alcohol) | 0%~1% 53%~63% 33%~43% |
| PSA | 99.38000 |
| LogP | |
| pH | 11.5-12.5 |
| Free Alcohol (wt %) | ≤1.0 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.512 |
| Viscosity (mPa·s), 20°C | 300-400 (at 20°C) |
Specific Gravity at 25°C [g/cm3] | 1.08-1.12 |
| Water (wt %) | 47-50 |
| DP | 1.3-1.5 |
| Surface tension (mN/m), 25°C, 0.1% | 28-30 |
| Microorganism (CFU/g) | ≤10 |
| Ash(wt%) | ≤3.0 |
 |  |
 |  |
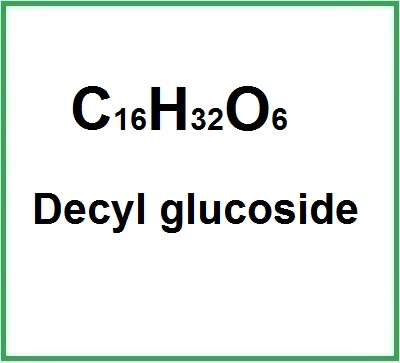
- Molecular Formula C16H32O6
- Molecular Weight 320.22 g/mol
- Exact Mass 320.219879
- CAS : 68515-73-1 141464-42-8 54549-25-6
- UNII
- EC Number 500-220-1
- DSSTox Substance ID DTXSID70872594
- MDL number
- PubChem Substance ID
- IUPAC (3R,4S,5S,6R)-2-decoxy-6-(hydroxymethyl)oxane-3,4,5-trio
- InChI=1S/C16H32O6/c1-2-3-4-5-6-7-8-9-10-21-16-15(20)14(19)13(18)12(11-17)22-16/h12-20H,2-11H2,1H3/t12-,13-,14+,15-,16?/m1/s1
- InChl Key JDRSMPFHFNXQRB-IWQYDBTJSA-N
- SMILES CCCCCCCCCCOC1C(C(C(C(O1)CO)O)O)O
- SCHEMBL 43196
Synonyms :
- Alkyl Polyglycoside
- APG
- Glucoside, decyl
- Decyl D-glucoside
- Decyl D-glucopyranosidee
- decyl-d-glucoside
- (3R,4S,5S,6R)-2-decoxy-6-(hydroxymethyl)oxane-3,4,5-triol
- (3R,4S,5S,6R)-2-(Decyloxy)-6-(hydroxymethyl)-tetrahydro-2H-Pyran-3,4,5-triol
References______________________________
(1) Kovačević AB, Müller RH, Keck CM. Formulation development of lipid nanoparticles: Improved lipid screening and development of tacrolimus loaded nanostructured lipid carriers (NLC). Int J Pharm. 2020 Feb 25;576:118918. doi: 10.1016/j.ijpharm.2019.118918
Abstract. Lipid nanoparticles are well-known nanocarriers for improved drug delivery. Their formulation development typically involves three formulations steps. In the first part a suitable lipid mixture which enables a high loading capacity and high encapsulation efficacy of the active needs to be identified (lipid screening). In the second step suitable stabilizers that enable the production of small-sized lipid nanoparticles with narrow size distribution and sufficient physical stability need to be identified (stabilizer screening, optimization of production parameters) and in the third step the biopharmaceutical efficacy needs to be evaluated. Based on the results obtained the formulations will require further optimization. The classical formulation development of lipid nanoparticles and especially the classical lipid screening is tedious. Therefore, in this study, a novel approach for the lipid screening that was based on the determination of the Hansen solubility parameters was evaluated and the results obtained were compared to the results from the classical model. Tacrolimus was used as a model drug. Results showed that both lipid screenings led to similar results, indicating that the new approach can be used for future developments. The optimized formulation was composed of a lipid matrix system that contained waxes, triglycerides and monoacylglycerols with various carbon chain lengths (C8, C10, C16, C18) and enabled an encapsulation efficiency of ~99%. The stabilizer screening showed that surfactants with high HLB values, lower molecular weight, and shorter alkyl chain length tended to form smaller particles with narrower size distribution and better physical stability. The most suitable surfactant was found to be a caprylyl/capryl glucoside (Plantacare® 810), a PEG-free stabilizer, that is extremely mild for atopic skin. It led to particle sizes of about 200 nm and a zeta potential well above |30| mV. The optimized formulation contained 0.1% tacrolimus and possessed good physical stability. In conclusion, an optimized method for the selection of lipids that results in a limited number of experiments could be established and tacrolimus loaded lipid nanoparticles with similar drug load as a marketed formulation was successfully developed in this study.
(2) Chung KH, Kim H, Park YK, Kim BH, Kim SJ, Jung SC. Decyl Glucoside Synthesized by Direct Glucosidation of D-Glucose Over Zeolite Catalysts and Its Estrogenicity as Non-Endocrine Disruptive Surfactant. J Nanosci Nanotechnol. 2019 Feb 1;19(2):1172-1175. doi: 10.1166/jnn.2019.15900
Abstract. The estrogenicity of decyl glucoside was asserted as a non-endocrine disruptive surfactant with its preparation method using zeolite catalysts. Its estrogenicity was estimated using E-assay method. The decyl glucoside was synthesized by direct glucosidation from D-glucose with 1-decanol. The conversion and yield were improved with increasing of amount of acid sites of the zeolite catalysts. The decyl glucopyranoside is more hydrophilic than nonylphenol and has a high wettability. The decyl glucopyranosides exhibited extremely lower proliferation of estrogenic cell compared with nonylphenol.
(3) Fiume MM, Heldreth B, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler D, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Safety assessment of decyl glucoside and other alkyl glucosides as used in cosmetics. Int J Toxicol. 2013 Sep-Oct;32(5 Suppl):22S-48S. doi: 10.1177/1091581813497764
Abstract. The Cosmetic Ingredient Review (CIR) Expert Panel assessed the safety of 19 alkyl glucosides as used in cosmetics and concluded that these ingredients are safe in the present practices of use and concentration when formulated to be nonirritating. Most of these ingredients function as surfactants in cosmetics, but some have additional functions as skin-conditioning agents, hair-conditioning agents, or emulsion stabilizers. The Panel reviewed the available animal and clinical data on these ingredients. Since glucoside hydrolases in human skin are likely to break down these ingredients to release their respective fatty acids and glucose, the Panel also reviewed CIR reports on the safety of fatty alcohols and were able to extrapolate data from those previous reports to support safety.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-11-06 16:45:48 | Chemical Risk: |