Cinnamyl Alcohol is a fragrance used in liquid soaps or shampoos to perfume the product.
It is a substance that must be reported on the label as it could give allergies and its maximum quantity is established by law.
Chemical modification of epidermal proteins by skin sensitizers is the molecular event which initiates the induction of contact allergy. However, not all chemical skin allergens react directly as haptens with epidermal proteins but need either a chemical (prehaptens) or metabolic (prohaptens) activation step to become reactive. Cinnamyl alcohol has been considered a model prohapten, as this skin sensitizer has no intrinsic reactivity. Therefore, the prevailing theory is that cinnamyl alcohol is enzymatically oxidized into the protein-reactive cinnamaldehyde, which is the sensitizing agent. Knowing that reconstructed human epidermis (RHE) models have been demonstrated to be quite similar to the normal human epidermis in terms of metabolic enzymes, use of RHE may be useful to investigate the in situ metabolism/activation of cinnamyl alcohol, particularly when coupled with high-resolution magic angle spinning nuclear magnetic resonance. Incubation of carbon-13 substituted cinnamyl derivatives with RHE did not result in the formation of cinnamaldehyde. The metabolites formed suggest the formation of an epoxy-alcohol and an allylic sulfate as potential electrophiles. These data suggest that cinnamyl alcohol is inducing skin sensitization through a route independent of the one involving cinnamaldehyde and should therefore be considered as a skin sensitizer on its own (1).
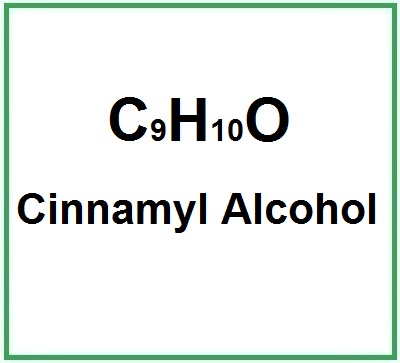
Molecular formula: C9H10O
Molecular weight: 134.18
CAS: 104-54-1 4407-36-7
Synonyms :
- cinnamic alcohol
- 3-phenyl-2-propene-1-ol
- 3-phenylprop-2-en-1-ol
References________________________________
(1) In Situ Metabolism of Cinnamyl Alcohol in Reconstructed Human Epidermis: New Insights into the Activation of This Fragrance Skin Sensitizer.
Moss E, Debeuckelaere C, Berl V, Elbayed K, Moussallieh FM, Namer IJ, Lepoittevin JP.
Chem Res Toxicol. 2016 Jul 18;29(7):1172-8. doi: 10.1021/acs.chemrestox.6b00148. Epub 2016 Jun 17.
![]() Cinnamyl alcohol
Cinnamyl alcohol