Hydroxycitronellal is a monoterpene, chiral molecule, a tertiary alcohol, found in the essential oils of aromatic plants of different species. Industrially, it is obtained by a process of boiling, filtration and condensation from both another monoterpene and from citronellal, a metabolite of plant origin.
Name breakdown and function of the components
- Hydroxy - Indicates the presence of a hydroxyl (OH) group in the compound.
- Citronellal - It's a molecule derived from citronella and has a structure similar to citronellol but with an aldehyde group.
Description and function of the raw materials used in production
Citronellal - It's the primary precursor in the synthesis of hydroxycitronellal and can be sourced from essential oils like citronella.
Summary of the industrial synthesis process step by step
- Hydroxylation - Citronellal undergoes a hydroxylation reaction to introduce a hydroxyl group into the molecule, forming hydroxycitronellal.
- Purification - The resulting product is then purified to remove any impurities or by-products.
- Isolation - Finally, hydroxycitronellal is separated and isolated as a pure compound.
It appears as a colourless or light yellow-white transparent liquid that is soluble in ethanol.

What it is used for and where
Hydroxycitronellal as a fragrance widely used by the cosmetics and food industries has been studied over a period of almost 25 years in a wide range of topics including toxicity, animal studies, safety assessment, and diagnostic evaluation by patch tests.
Medical
Synthetic fragrance with antifungal activity (1) against fungi of the Candida genus especially in oral cavity infections not involving Albicans strains.
Cosmetics
Hydroxycitronellal is used as a fragrance to obtain the scents of lilac, lily of the valley, jasmine, lemon, citrus and various fruits obtained chemically and as an antifungal to preserve the product from moulds, but is considered a sensitising allergen in human skin.
Hydroxycitronellal is a restricted III/72 as a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009.The presence of the substance must be indicated in the list of ingredients referred to in Article 19(1)(g) when its concentration exceeds: — 0,001 % in leave-on products — 0,01 % in rinse-off products. Maximum concentration in ready for use preparation 1,0 %.
Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.
Doses: typical inclusion levels are between
- 0.015-0.478% in perfumes
- 0.1-3.6% creams and lotions
Food
Raw material for creating fragrances and flavourings in foods
Other uses
Solvent, synthetic organic intermediate, spices, household hygiene products. Hydroxycitronellal derivatives have proved useful as insect repellents.
Safety
Hydroxycitronellal is considered an allergen, the effects of which may be manifested by an increase in histamines. Sensitisation can occur even at lower concentrations (2). A study conducted in the Netherlands with the help of K. Malten revealed that 10% of all positive test reactions among dermatitis cases were attributable to Hydroxycitronellal (3).
Hydroxycitronellal studies
| Appearance | Yellow transparent liquid |
| Boiling Point | 251.6±23.0°C at 760 mmHg |
| Melting Point | 19°C |
| Flash Point | 103.8±15.2°C |
| Density | 0.9±0.1 g/cm3 |
| Refraction Index | 1.443 |
| Vapor Pressure | 0.0±1.1 mmHg at 25°C |
| PSA | 37.30000 |
| LogP | 1.54 |
| Storage | Room temperature |
| Shelf Life | 2 years |
Chemical Safety
|  |
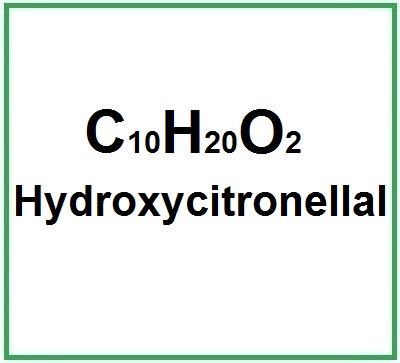
- Molecular Formula C10H20O2
- Molecular Weight 172.26
- Exact Mass 172.146332
- CAS 107-75-5
- UNII 8SQ0VA4YUR
- EC Number 203-518-7
- DSSTox Substance ID DTXSID6042232
- IUPAC 7-hydroxy-3,7-dimethyloctanal
- InChl=1S/C10H20O2/c1-9(6-8-11)5-4-7-10(2,3)12/h8-9,12H,4-7H2,1-3H3
- InChl Key WPFVBOQKRVRMJB-UHFFFAOYSA-N
- SMILES CC(CCCC(C)(C)O)CC=O
- MDL number MFCD00014681
- PubChem Substance ID 24901168 57652938
- ChEBI 53459
- RTECS RG7850000
- JECFA 611
- FEMA 2583
- NACRES NA.52 NA.21
- NSC 406740 163509
- Council of Europe no.100
Synonyms:
- 7-Hydroxy-3,7-dimethyl-1-octanal
- 7-Hydroxy-3,7-dimethyloctanal
- 7-hydroxycitronellal
- Hydroxycitronellylidene methyl anthranilate
References_____________________________________________________________________
(1) Oliveira Filho, A. A., Oliveira, H. M. B. F. D., Medeiros, C. I. S., Pessôa, H. D. L. F., Siqueira Júnior, J. P. D., & Lima, E. D. O. (2017). Antifungal effect of 7-hydroxycitronellal against C. Tropicalis strains: an in vitro approach. Biosci. j.(Online), 204-208.
(2) Ford RA, Api AM, Suskind RR. Allergic contact sensitization potential of hydroxycitronellal in humans. Food Chem Toxicol. 1988 Nov-Dec;26(11-12):921-6. doi: 10.1016/0278-6915(88)90090-7.
Abstract. Hydroxycitronellal, an important ingredient in fragrances, was studied for its sensitizing potential in human skin. Fifteen human maximization tests were conducted with hydroxycitronellal obtained from four different sources at induction concentrations from 5 to 12%. No reactions were induced at 5% in two separate panels while 10% sensitized 2/25 panelists in one test but none in a second. Induction at 12% produced sensitization in 8 of 11 tests. Impurities do not appear to be a sensitizing factor. There is some evidence that the l-stereoisomer is a less potent sensitizer than the d-stereoisomer. In an initial modified human repeat-insult patch-test two positive reactions to challenge were observed among 197 panelists, one at a concentration of 5% and the other at 7.5%. When 100 of the non-reacting panelists were re-exposed in the same way, allergic sensitization reactions appeared during the induction period with concentrations as low as 2.5%. When 28 sensitized panelists were exposed to 1% concentrations in a simulated use test, there were three reactors. A no-effect level for sensitization has not been determined although the lowest concentrations tested were in the product usage range.
(3) Suskind, R. R. (1998). The hydroxycitronellal story: what can we learn from it?. In Fragrances (pp. 159-165). Springer, Berlin, Heidelberg.
![]() Hydroxycitronellal
Hydroxycitronellal