Hexyl Salicylate is a chemical compound, an ester of salicylic acid, is a fragrance that is added to liquid soaps, shampoos etc. to perfume them.
It is an ester derived from salicylic acid and hexyl alcohol. It is primarily used in cosmetics and personal care products for its fragrance and conditioning properties. With its mildly sweet and fruity floral scent, Hexyl Salicylate is commonly employed as a fragrance component in perfumes, deodorants, creams, and lotions.
Chemical Composition and Structure
Hexyl Salicylate is an ester formed by the reaction between salicylic acid and hexanol (hexyl alcohol). Its chemical structure consists of a salicylate group attached to a six-carbon alkyl chain, giving the compound its fragrance properties and moderate solubility in oils.
Physical Properties
Hexyl Salicylate is typically a colorless or slightly yellow liquid, soluble in oils and slightly soluble in water. It has a delicate, floral, and fruity scent, making it a popular choice in personal care products. Its moderate volatility makes it suitable for use in long-lasting fragrances.

Production Process
Hexyl Salicylate is produced through an esterification reaction between salicylic acid and hexanol. This esterification process removes a water molecule, creating a stable ester bond between the acid and alcohol, resulting in a fragrant and stable compound.
The name describes the structure of the molecule:
- Hexyl refers to the hexyl group, an alkyl group derived from hexane, which is a hydrocarbon with six carbon atoms. The hexyl group imparts certain olfactory properties to the compound.
- Salicylate refers to the salt or ester of salicylic acid. Salicylates are known for their properties as UV filters and for their aromatic qualities.
Raw Materials and Their Functions
Salicylic Acid. An organic acid used as the base for the production of hexyl salicylate. It contributes to the UV-absorbing properties of the compound.
Hexanol (Hexyl Alcohol). A fatty alcohol used to esterify salicylic acid, imparting aromatic properties to the compound.
Industrial Chemical Synthesis of Hexyl Salicylate
- Esterification with the reaction of salicylic acid with hexanol. During this reaction, an ester bond is formed between salicylic acid and hexanol, producing hexyl salicylate.
- Reaction Control. The esterification reaction is monitored to ensure it occurs correctly and the final product has the desired properties.
- Purification. After the reaction, hexyl salicylate is purified to remove impurities and by-products.
- Quality Control. The purified hexyl salicylate undergoes quality checks to ensure it meets the required standards. After quality control, it is packaged for use in cosmetic and skincare products, where it utilizes its properties as a fragrance and UV filter.
Form and Color
Hexyl Salicylate is typically a liquid. This compound is usually colorless or very light in color.

What it is used for and where
Salicylic Esters are a class of highly used chemicals such as aromas and fragrances in foods, beverages and many consumer products.
Hexyl Salicylate is primarily used as a fragrance in cosmetic and personal care products, such as perfumes, lotions, and body cleansing products. Hexyl Salicylate is known for its floral and slightly herbaceous scent, contributing to creating pleasant and long-lasting fragrances. It is also used in some sunscreen formulations for its UV-absorbing properties.
Cosmetics
Fragrance. It plays a decisive and important role in the formulation of cosmetic products as it provides the possibility of enhancing, masking or adding fragrance to the final product, increasing its marketability. It is able to create a perceptible pleasant odour, masking a bad smell. The consumer always expects to find a pleasant or distinctive scent in a cosmetic product.
Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.
Safety
Health and Safety Considerations
Safety in Use
Hexyl Salicylate is generally considered safe for use in cosmetic products. However, as with many fragrance ingredients, it may cause skin irritation in individuals with sensitive or allergic skin. It should be used within recommended concentrations, especially in skincare and haircare products.
Allergic Reactions
In rare cases, Hexyl Salicylate can cause allergic reactions or skin irritation, particularly in individuals with sensitive skin. It is advisable to perform a patch test before using products containing this ingredient.
Toxicity and Carcinogenicity
It is considered safe for use in cosmetics at regulated concentrations set by international guidelines.
Environmental and Safety Considerations
Hexyl Salicylate is an organic compound that can biodegrade in the environment. However, as with many chemical compounds, it is important to properly dispose of products containing it to avoid excessive accumulation in the environment, particularly in aquatic systems.
Regulatory Status
Hexyl Salicylate is regulated by the European Union and the Food and Drug Administration (FDA) in the United States. It is approved for use in cosmetic and personal care products, provided it is used within the safe concentration limits set by current regulations.
Studies
However, some personal care products are polluting : Benzyl Salicylate, Hexyl Salicylate, Lemonile and Okoumal were detected for the first time in Antarctic natural seawater, and reached total concentrations up to 100ngL-1 (1).
Hexyl Salicylate studies
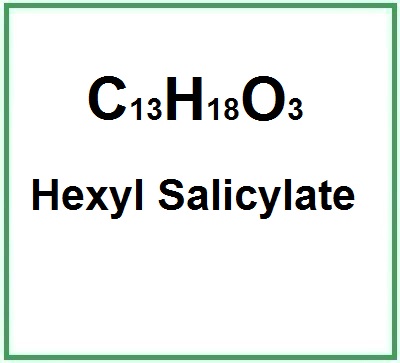
Molecular Formula : C13H18O3
Molecular Weight : 222.284 g/mol
CAS : 6259-76-3
EINECS 228-408-6
Synonyms :
- Hexyl 2-hydroxybenzoate
- Benzoic acid, 2-hydroxy-, hexyl ester
- 1-Hexyl salicylate
- Hexyl salicylic acid
- Salicylic acid, hexyl ester
- Hexyl 2-hydroxybenzoic acid
References___________________________________________________________________
(1) Vecchiato M, Gregoris E, Barbaro E, Barbante C, Piazza R, Gambaro A. Fragrances in the seawater of Terra Nova Bay, Antarctica. Sci Total Environ. 2017 Sep 1;593-594:375-379. doi: 10.1016/j.scitotenv.2017.03.197.
Abstract. Personal Care Products are emerging pollutants whose distribution in the Antarctic and remote environments is still largely unknown. Among PCPs, long-lasting and stable Fragrance Materials were selected to perform a first pilot study on their occurrence in the coastal surface seawater of Terra Nova Bay in the Ross Sea, Antarctica. Ambrofix, Amyl Salicylate, Benzyl Salicylate, Hexyl Salicylate, Lemonile and Okoumal were detected for the first time in Antarctic natural seawater, and reached total concentrations up to 100ngL-1. Treated discharges from the Italian research station Mario Zucchelli (MZS) contain FMs, however concentrations in nearby Tethys Bay increase during the seasonal melt of the sea ice and its snow cover: variability in emissions and distribution, as well as a contribution from atmospheric (long or short-range) transport were hypothesized.
![]() Hexyl Salicylate
Hexyl Salicylate