![]() Calcium carbonate
Calcium carbonate
Rating : 7.5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from Handy23
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Calcium carbonate studies" about Calcium carbonate Review Consensus 10 by Handy23 (4274 pt) | 2022-Jun-28 17:22 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Lynch RJ, ten Cate JM. The anti-caries efficacy of calcium carbonate-based fluoride toothpastes. Int Dent J. 2005;55(3 Suppl 1):175-8. doi: 10.1111/j.1875-595x.2005.tb00055.x.
Abstract. To summarise clinical support for the anti-caries efficacy of fluoride toothpastes containing sodium monofluorophosphate (SMFP) and to discuss the possible means by which the abrasive particles in calcium carbonate-based SMFP toothpastes might complement and/or enhance fluoride efficacy.
McClelland SC, Cassey P, Maurer G, Hauber ME, Portugal SJ. How much calcium to shell out? Eggshell calcium carbonate content is greater in birds with thinner shells, larger clutches and longer lifespans. J R Soc Interface. 2021 Sep;18(182):20210502. doi: 10.1098/rsif.2021.0502.
Abstract. The avian eggshell is a bio-ceramic structure that protects the embryo. It is composed almost entirely of calcium carbonate and a small amount of organic material. An optimal amount of calcium carbonate in the eggshell is essential for the embryo's development, yet how the ratio of calcium carbonate to organic matter varies between species has not been investigated. Calcium is a limiting resource for most birds, so its investment in their eggs should be optimized for a bird's life history. We measured the relative calcium carbonate content of eggshells in 222 bird species and tested hypotheses for how this trait has evolved with the life-history strategies of these species and other traits of their respective egg physiologies. We found that (i) eggshell calcium carbonate content was positively correlated with species having thinner eggshells and smaller than expected eggs relative to incubating parental mass, (ii) species with small mean clutch sizes had lower calcium carbonate content in their eggshells, and (iii) for species with larger clutch sizes, eggshell calcium carbonate content was negatively correlated with their mean lifespan. The pattern of lower eggshell calcium carbonate in longer lived, larger clutched birds suggests that calcium provision to the eggshell has long-term costs for the individual.
Meyer C, Cameron K, Battistella M. New agent to treat elevated phosphate levels: magnesium carbonate/calcium carbonate tablets. CANNT J. 2012 Oct-Dec;22(4):33-5; quiz 36-7.
Abstract. In summary, Binaphos CM, a magnesium carbonate/calcium carbonate combination phosphate binder, is marketed for treating elevated phosphate levels in dialysis patients. Although studies using magnesium/calcium carbonate as a phosphate binder are short term with small numbers of patients, this phosphate binder has shown some promising results and may provide clinicians with an alternative for phosphate binding. Using a combination phosphate binder may reduce pill burden and encourage patient compliance. In addition to calcium and phosphate, it is imperative to diligently monitor magnesium levels in patients started on this medication, as magnesium levels may increase with longer duration of use. Additional randomized controlled trials are necessary to evaluate long-term efficacy and safety of this combination phosphate binder.
Hargis CW, Chen IA, Devenney M, Fernandez MJ, Gilliam RJ, Thatcher RP. Calcium Carbonate Cement: A Carbon Capture, Utilization, and Storage (CCUS) Technique. Materials (Basel). 2021 May 21;14(11):2709. doi: 10.3390/ma14112709.
Abstract. A novel calcium carbonate cement system that mimics the naturally occurring mineralization process of carbon dioxide to biogenic or geologic calcium carbonate deposits was developed utilizing carbon dioxide-containing flue gas and high-calcium industrial solid waste as raw materials. The calcium carbonate cement reaction is based on the polymorphic transformation from metastable vaterite to aragonite and can achieve >40 MPa compressive strength. Due to its unique properties, the calcium carbonate cement is well suited for building materials applications with controlled factory manufacturing processes that can take advantage of its rapid curing at elevated temperatures and lower density for competitive advantages. Examples of suitable applications are lightweight fiber cement board and aerated concrete. The new cement system described is an environmentally sustainable alternative cement that can be carbon negative, meaning more carbon dioxide is captured during its manufacture than is emitted.
Cao M, Ming X, He K, Li L, Shen S. Effect of Macro-, Micro- and Nano-Calcium Carbonate on Properties of Cementitious Composites-A Review. Materials (Basel). 2019 Mar 7;12(5):781. doi: 10.3390/ma12050781.
Abstract. Calcium carbonate is wildly used in cementitious composites at different scales and can affect the properties of cementitious composites through physical effects (such as the filler effect, dilution effect and nucleation effect) and chemical effects. The effects of macro (>1 mm)-, micro (1 μm⁻1 mm)- and nano (<1 μm)-sizes of calcium carbonate on the hydration process, workability, mechanical properties and durability are reviewed. Macro-calcium carbonate mainly acts as an inert filler and can be involved in building the skeletons of hardened cementitious composites to provide part of the strength. Micro-calcium carbonate not only fills the voids between cement grains, but also accelerates the hydration process and affects the workability, mechanical properties and durability through the dilution, nucleation and even chemical effects. Nano-calcium carbonate also has both physical and chemical effects on the properties of cementitious composites, and these effects behave even more effectively than those of micro-calcium carbonate. However, agglomeration of nano-calcium carbonate reduces its enhancement effects remarkably.
Cartwright JH, Checa AG, Gale JD, Gebauer D, Sainz-Díaz CI. Calcium carbonate polyamorphism and its role in biomineralization: how many amorphous calcium carbonates are there? Angew Chem Int Ed Engl. 2012 Nov 26;51(48):11960-70. doi: 10.1002/anie.201203125.
Abstract. Here we summarize what is known about polyamorphism in calcium carbonate as well as what is understood about the role of amorphous calcium carbonate in biominerals.
Stringer MD, Soloway RD, Taylor DR, Riyad K, Toogood G. Calcium carbonate gallstones in children. J Pediatr Surg. 2007 Oct;42(10):1677-82. doi: 10.1016/j.jpedsurg.2007.05.022.
Abstract. In the United States, cholesterol stones account for 70% to 95% of adult gallstones and black pigment stones for most of the remainder. Calcium carbonate stones are exceptionally rare. A previous analysis of a small number of pediatric gallstones from the north of England showed a remarkably high prevalence of calcium carbonate stones. The aims of this study were to analyze a much larger series of pediatric gallstones from our region and to compare their chemical composition with a series of adult gallstones from the same geographic area.
Tawbi HA, Tran AL, Christner SM, Lin Y, Johnson M, Mowrey E, Appleman LR, Stoller R, Miller BM, Egorin MJ, Beumer JH. Calcium carbonate does not affect nilotinib pharmacokinetics in healthy volunteers. Cancer Chemother Pharmacol. 2013 Nov;72(5):1143-7. doi: 10.1007/s00280-013-2283-x.
Abstract. Nilotinib is a second-generation oral tyrosine kinase inhibitor with superior efficacy compared with imatinib mesylate in the treatment for chronic phase chronic myelogenous leukemia. Calcium carbonate is commonly used as a source of calcium supplementation or as antacid to ameliorate the gastrointestinal side effects associated with nilotinib, which could have unknown effects on nilotinib absorption. The purpose of this study was to provide information on the effect of calcium carbonate on the PK of nilotinib in healthy volunteers.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Calcium carbonate Review Consensus 10 by Handy23 (4274 pt) | 2023-Jul-15 16:33 |
| Read the full Tiiip | (Send your comment) |
Calcium carbonate inorganic compound, is one of the most common minerals found in rock agglomerates throughout the world and consists mainly of: calcium, carbon, oxygen. It is the primary component of eggshells, snails, pearls and marine organisms. Calcium carbonate is the active ingredient in agricultural lime and is created when calcium ions in hard water react with carbonate ions to create limescale.
The name "calcium carbonate" is derived from its chemical composition: one calcium ion combined with one carbonate ion.
Calcium carbonate is not typically synthesized in a laboratory or industrial setting because it is readily available in nature. However, it can be synthesized by mixing solutions of calcium ions and carbonate ions. The resulting precipitate is calcium carbonate.
Here is a simple example of how this can be done:
- Preparation of solutions. A solution of calcium chloride (CaCl2) and another solution of sodium carbonate (Na2CO3). These are the sources of the calcium ions and carbonate ions, respectively.
- Mixing of solutions. A sodium carbonate solution is added to the calcium chloride solution while the compounds are mixed.
- Formation of precipitate. As the solutions mix, a precipitate of calcium carbonate forms. The reaction is: CaCl2 + Na2CO3 -> CaCO3 + 2NaCl.
- Isolation of product. The calcium carbonate precipitate can be collected by filtration, then washed and dried to obtain the pure compound.
It appears in the form of a white powder insoluble in water colorless, odorless and tasteless.

What it is used for and where
Food
Ingredient included in the list of European food additives as dye E170.
Constructions (cement, plasters, asphalt).
The use of calcium carbonate precipitate protect concrete surface against the ingress of harmful external agents (1).
Medical
The synthesised biobased Calcium carbonate nanocrystals had demonstrated to be an effective carrier for delivery of anticancer drug doxorubicin (DOX). Findings suggest that Calcium carbonate nanocrystals hold tremendous promise in the areas of controlled drug delivery and targeted cancer therapy (2).
Dentin hypersensitivity reduction in adults with a clinical diagnosis of dentin hypersensitivity (3).and indicated as a treatment in cases of calcium deficiency in elderly subjects and in combination with therapies for osteoporosis (4). Calcium carbonate is able to increase calcium levels and neutralise plaque acids (5).
Cosmetics
It is a restricted ingredient as IV/124 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009.
- Abrasive agent. It contains abrasive particles to remove stains or biofilm that accumulate on the stratum corneum or teeth. Baking soda, kieselguhr, silica and many others have abrasive properties. Peeling or exfoliating products used in dermatology or cosmetic applications contain abrasive agents in the form of synthetic microspheres, however these microspheres or abrasive particles are not biodegradable and create pollution in aquatic ecosystems.
- Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
- Bulking agent. It regulates the water content, dilutes other solids, can increase the volume of a product for better flow, acts as a buffer against organic acids, helps to keep the pH of the mixture within a certain level.
- Opacifying agent. It is useful into formulations that may be translucent or transparent to make them opaque and less permeable to light.
- Oral care agent. This ingredient can be placed in the oral cavity to improve and/or maintain oral hygiene and health, to prevent or improve a disorder of the teeth, gums, mucous membrane. It provides cosmetic effects to the oral cavity as a protector, cleanser, deodorant.
Safety. It is an ingredient that has no particular health warnings and can therefore be used in all cosmetic products.
and is widely used by industries:
- Adhesives, sealants
- Rugs
- Environment (process of desulfurization of combustion gases)
- Fertilizers
- Food and pharmaceutical
- Glass and ceramics
- Products for the home
- Paints and surface coatings
- Paper
- Plastics and composites
- Rubber and elastomers
For more information:
Typical commercial product characteristics Calcium carbonate
| Appearance | White powder |
| pH | 9.0-10.5 |
| Boiling Point | 333.6ºC at 760mmHg |
| Melting Point | 825°C |
| Flash Point | 197ºC |
| Density | 2.93 g/mL at 25 °C(lit.) |
| Refraction Index | 1.6583 |
| PSA | 63.19000 |
| HCL insoluble content | ≤0.20% |
| Volatile content below 105℃ | ≤1.00 |
| Free Alkali | ≤0.10% |
| Whiteness degree | ≥90% |
| Oil Absorption | 50-60ml/g |
| Sedimentation volume | ≥2.2-2.8 ml/g |
| Fe | ≤0.12% |
| Mn | ≤0.01% |
| Average Particle | 0.5-15um |
| Mesh | 400/800/1000/1250/1500 |
| Safety | 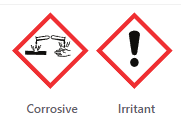 |
 |  |
 |  |
- Molecular Formula CCaO3 CaCO3
- Molecular Weight 100.086 g/mol
- Exact Mass 101.962982
- CAS 471-34-1
- UNII H0G9379FGK
- EC Number 207-439-9
- DSSTox Substance ID DTXSID3036238 DTXSID9050486 DTXSID90109374 DTXSID80163829
- IUPAC calcium;carbonate
- InChI=1S/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
- InChl Key VTYYLEPIZMXCLO-UHFFFAOYSA-L
- SMILES C(=O)([O-])[O-].[Ca+2]
- MDL number MFCD00010906
- PubChem Substance ID 329749202
- ChEBI 3311
- ICSC 1193
- RXCUI 1897
- RTECS EV9580000 FF9335000
- Beilstein 8008338
- NCI C332
Synonyms :
- CI 77220
- Calcite (Ca(Co3))
- Aragonite
- Cupric carbonate
- C50 (carbonate)
- Carbonic acid, calcium salt (1:1)
References____________________________________________________________________
(1) Van Tittelboom K, De Belie N, De Muynck W, Verstraete W. 2010. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 40:157–166. 10.1016/j.cemconres.2009.08.025
(2) Shafiu Kamba A, Ismail M, Tengku Ibrahim TA, Zakaria ZA. A pH-sensitive, biobased calcium carbonate aragonite nanocrystal as a novel anticancer delivery system. Biomed Res Int. 2013;2013:587451. doi: 10.1155/2013/587451.
(3) Collins JR, Richardson D, Sotero K, Mateo LR, Mauriz I. Beneficial effects of an arginine-calcium carbonate desensitizing paste for treatment of dentin hypersensitivity. Am J Dent. 2013 Apr;26(2):63-7.
(4) Wang J, Tao S, Jin X, Song Y, Zhou W, Lou H, Zhao R, Wang C, Hu F, Yuan H. Calcium Supplement by Tetracycline guided amorphous Calcium Carbonate potentiates Osteoblast promotion for Synergetic Osteoporosis Therapy. Theranostics. 2020 Jul 9;10(19):8591-8605. doi: 10.7150/thno.45142.
(5) Lynch RJ, ten Cate JM. The anti-caries efficacy of calcium carbonate-based fluoride toothpastes. Int Dent J. 2005;55(3 Suppl 1):175-8. doi: 10.1111/j.1875-595x.2005.tb00055.x.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-03-27 19:09:28 | Chemical Risk: |


