![]() Sodium ascorbate
Sodium ascorbate
Rating : 7.3
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antioxidant (1) Antidiabetic (1)15 pts from Nat45
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Sodium ascorbate studies" about Sodium ascorbate Review Consensus 8 by MGannelly (1342 pt) | 2019-Oct-19 21:12 |
| Read the full Tiiip | (Send your comment) |
Best studies on sodium ascorbate
BACKGROUND:Vitamin C (L-ascorbic acid), a known enhancer of collagen deposition, has also been identified as an inhibitor of elastogenesis. OBJECTIVE: Present studies explored whether and how the L-ascorbic acid derivative (+) sodium L-ascorbate (SA) would affect production of collagen and elastic fibers in cultures of fibroblasts derived from normal human skin and dermal fat, as well as in explants of normal human skin, stretch-marked skin and keloids. METHODS: Effects of SA on the extracellular matrix production were assessed quantitatively by PCR analyses, western blots, biochemical assay of insoluble elastin and by immuno-histochemistry. We also evaluated effects of SA on production of the reactive oxygen species (ROS) and phosphorylation of IGF-I and insulin receptors. RESULTS: SA, applied in 50-200 μM concentrations, stimulates production of both collagen and elastic fibers in all tested cultures. Moreover, combination of SA with a proline hydroxylase inhibitor induces a beneficial remodelling in explants of dermal scars, resulting in the inhibition of collagen deposition and induction of new elastogenesis. Importantly, we revealed that SA stimulates elastogenesis only after intracellular influx of non-oxidized ascorbate anions (facilitated by the sodium-dependent ascorbate transporter), that causes reduction of intracellular ROS, activation of c-Src tyrosine kinase and the enhancement of IGF-1-induced phosphorylation of the IGF-1 receptor that ultimately triggers elastogenic signalling pathway. CONCLUSION:
Our results endorse the use of this potent stimulator of collagen and elastin production in the treatment of wrinkled and stretch-marked skin. They also encourage inclusion of SA into therapeutic combinations with collagenogenesis inhibitors to prevent formation of dermal scars and keloids (1).
There is mounting evidence demonstrating causative links between hyperglycemia, oxidative stress, and insulin resistance, the core pathophysiological features of type 2 diabetes mellitus. Using a combinational approach, we synthesized a vanadium-antioxidant (i.e., l-ascorbic acid) complex and examined its effect on insulin resistance and oxidative stress. This study was designed to examine whether vanadyl(IV)-ascorbate complex (VOAsc) would reduce oxidative stress, hyperglycemia, and insulin resistance in high-fat high-sucrose diet (HFSD)-induced type 2 diabetes in mice. Male C57BL/6J mice were fed a HFSD for 12 weeks to induce insulin resistance, rendering them diabetic. Diabetic mice were treated with rosiglitazone, sodium l-ascorbate, or VOAsc. At the end of treatment, fasting blood glucose, fasting serum insulin, homeostasis model assessment-insulin resistance index, and serum adipocytokine levels were measured. Serum levels of nitric oxide (NO) parameters were also determined. The liver was isolated and used for determination of malondialdehyde, reduced glutathione, and catalase levels, and superoxide dismutase and glutathione peroxidase activities. VOAsc groups exhibited significant reductions in serum adipocytokine and NO levels, and oxidative stress parameters compared to the corresponding values in the untreated diabetic mice. The results indicated that VOAsc is non-toxic. In conclusion, we identified VOAsc as a potentially effective adjunct therapy for the management of type 2 diabetes (2).
A comparative study on the antioxidant effect of rosemary extract (RE) and sodium ascorbate (SA) on lipid and colour oxidation of liver pâté made of lard and pork liver was done. During the 48 hour experimental time all the pâtés were wrapped in a foil and stored in cold room of 3.5°C under light of 1000 lux. Colour stability was monitored by instrumental colour measurement (CIE L*a*b* colour space) whereas lipid stability was measured by the determination of the 2-thiobarbituric acid reactive substances (TBARS). In the present study RE doses range (0,125, 250, 375 and 500 ppm) showed no significant (p>0.05) and linear effect on colour stability. However thez RE revealed a significant effect (p<0.05) against lipid oxidation and linearly reduces the TBARS number. The added SA doses (0, 250, 500, 750 and 1000 ppm) revealed significant (p<0.05) and linear effect in reducing discoloration. However the studied SA dose ranges showed no significant (p>0.05) effect on TBARS number. In this study RE was showed better performance against lipid oxidation and SA was potent against discoloration. The effect of the added spices used in manufacturing of the studied product showed no significant (p>0.05) effect against lipid and color oxidation. However the added spices revealed possible antagonistic and synergetic relationship with the studied the antioxidant systems (RE & SA) (3).
BACKGROUND: Neuroblastoma (NB) is an extra-cranial solid tumour of childhood. In spite of the good clinical response to first-line therapy, complete eradication of NB cells is rarely achieved. Thus, new therapeutic strategies are needed to eradicate surviving NB cells and prevent relapse. Sodium ascorbate has been recently reported to induce apoptosis of B16 melanoma cells through down-regulation of the transferrin receptor, CD71. Since NB and melanoma share the same embryologic neuroectodermal origin, we used different human NB cell lines to assess whether the same findings occurred. RESULTS: We could observe dose- and time-dependent induction of apoptosis in all NB cell lines. Sodium ascorbate decreased the expression of CD71 and caused cell death within 24 h. An increase in the global and specific caspase activity took place, as well as an early loss of the mitochondrial transmembrane potential. Moreover, intracellular iron was significantly decreased after exposure to sodium ascorbate. Apoptotic markers were reverted when the cells were pretreated with the iron donor ferric ammonium citrate (FAC), further confirming that iron depletion is responsible for the ascorbate-induced cell death in NB cells. CONCLUSION: Sodium ascorbate is highly toxic to neuroblastoma cell lines and the specific mechanism of vitamin C-induced apoptosis is due to a perturbation of intracellular iron levels ensuing TfR-downregulation (4).
The bioavailability of ascorbic acid from food has been assumed to be high, but little quantitative information has been available to substantiate this assumption because of the limited precision and low statistical power of previous studies. A recent depletion-repletion study with a more effective experimental design has shown clearly that ascorbic acid bioavailability is equivalent in ascorbic acid tablets (with and without iron), orange juice, whole orange sections, and cooked broccoli. The bioavailability of ascorbic acid in raw broccoli was 20% lower, although this difference would probably have little nutritional significance in typical mixed diets (5).
References________________________________________
(1) Sodium L-ascorbate enhances elastic fibers deposition by fibroblasts from normal and pathologic human skin.
Hinek A, Kim HJ, Wang Y, Wang A, Mitts TF.
J Dermatol Sci. 2014 Sep
(2) Ameliorative effect of vanadyl(IV)-ascorbate complex on high-fat high-sucrose diet-induced hyperglycemia, insulin resistance, and oxidative stress in mice.
Liu Y, Xu J, Guo Y, Xue Y, Wang J, Xue C.
J Trace Elem Med Biol. 2015 Oct
(3) A comparative study on the effect of rosemary extract and sodium ascorbate on lipid and pigment oxidative stability of liver pate
Demewez Moges Haile
J Food Sci Technol. 2015 Feb; 52(2): 992–999. Published online 2013 Jul 7. doi: 10.1007/s13197-013-1087-7
(4) Sodium Ascorbate induces apoptosis in neuroblastoma cell lines by interfering with iron uptake
Roberta Carosio, Guendalina Zuccari, Isabella Orienti, Salvatore Mangraviti, Paolo G Montaldo
Mol Cancer. 2007; 6: 55. Published online 2007 Aug 30. doi: 10.1186/1476-4598-6-55
(5) Gregory JF., 3rd Ascorbic acid bioavailability in foods and supplements. Nutr Rev. 1993;51:301–303
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Sodium ascorbate Review Consensus 15 by Nat45 (5724 pt) | 2025-Jan-07 14:30 |
| Read the full Tiiip | (Send your comment) |
Sodium Ascorbate, the sodium salt of ascorbic acid (vitamin C), is a water-soluble compound widely used in cosmetics, food, and pharmaceuticals. Known for its antioxidant properties, it protects the skin from oxidative stress, supports collagen synthesis, and brightens the skin tone. In formulations, it acts as a stabilizer and preservative, making it a versatile ingredient in skincare and anti-aging products.
Chemical Composition and Structure
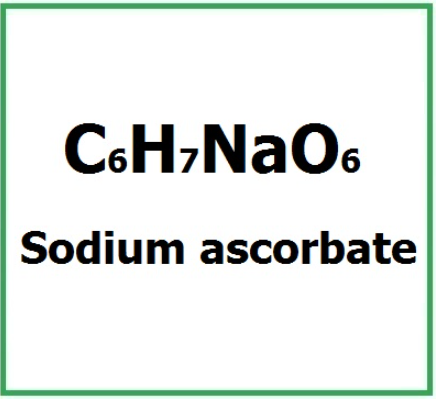
Sodium Ascorbate has the chemical formula C6H7NaO6.
Core Structure:
- Derived from ascorbic acid by neutralizing one of its acidic groups with sodium, enhancing stability and water solubility.
Properties:
- Retains the antioxidant properties of ascorbic acid.
- Exhibits reduced acidity, making it gentler on the skin and stomach compared to pure vitamin C.
Physicochemical Properties
- Appearance: White to pale yellow crystalline powder.
- Odor: Odorless.
- Solubility: Highly soluble in water.
- Stability: More stable than ascorbic acid in aqueous solutions, though sensitive to heat, light, and air.
- pH: Neutral to slightly alkaline in aqueous solutions.
Production Process
Neutralization:
- Ascorbic acid reacts with sodium carbonate or sodium hydroxide to form sodium ascorbate.
Purification:
- The resulting solution is purified through filtration and recrystallization to remove impurities.
Drying and Packaging:
- The purified product is dried and packaged under controlled conditions to preserve its antioxidant properties.
Applications
Medical Applications
Vitamin C Supplementation:
- Used in oral and injectable forms to prevent and treat vitamin C deficiencies.
- Supports immune function and wound healing.
Antioxidant Therapy:
- Reduces oxidative stress in chronic conditions and promotes recovery.
Cosmetics
Antioxidant Protection:
- Protects the skin from free radical damage caused by UV radiation and environmental stressors.
Collagen Synthesis:
- Promotes collagen production, improving skin elasticity and reducing the appearance of fine lines and wrinkles.
Brightening Effect:
- Reduces melanin production, helping to lighten dark spots and even out skin tone.
Stabilizer:
- Used to stabilize other active ingredients in formulations, enhancing product longevity.
Food Applications
- Acts as a preservative in food products, preventing oxidation and maintaining freshness.
- Used as a vitamin C source in fortified foods and beverages.
Industrial Applications
- Used in formulations requiring a stable, water-soluble antioxidant.
- Found in natural and organic product lines due to its multifunctional benefits.
Environmental and Safety Considerations
Biodegradability:
- Sodium Ascorbate is biodegradable and poses no significant environmental risk.
Safety Profile:
- Considered safe for cosmetic and pharmaceutical use within regulated concentrations.
- Non-irritating and suitable for sensitive skin formulations.
Sustainability:
- Derived from naturally occurring ascorbic acid, with low environmental impact during production.
Studies
Food
It is an antioxidant used mostly in the food sector where it is labeled with the number E301 in food additives.
Medical
In dentistry it is used in teeth whitening (1) and as a binder against micro-infiltration (2).
Cosmetics
Antioxidant agent. Ingredient that counteracts oxidative stress and prevents cell damage. Free radicals, pathological inflammatory processes, reactive nitrogen species and reactive oxygen species are responsible for the ageing process and many diseases caused by oxidation.
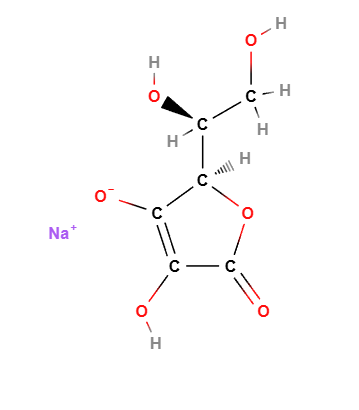 |  |
Molecular Formula : C6H7O6Na C6H7NaO6
Molecular Weight: 198.106 g/mol
CAS: 134-03-2 58657-35-5
EC Number: 205-126-1
UNIII: S033EH8359
Synonyms:
- L-Ascorbic acid sodium salt
- L-Ascorbic acid, monosodium salt
- Vitamin C sodium
- E301
- Monosodium L-ascorbate
- Ascorbicin
- Ascorbic acid sodium salt
- Sodascorbate
- Ascorbin
- Cebitate
- Ascorbic acid sodium derivative
- Aminofenitrooxon
- Sodium derivative of 3-oxo-L-gulofuranolactone
- Natrii ascorbas
- sodium (2R)-2-[(1S)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2,5-dihydrofuran-3-olate
- Sodium (R)-2-((S)-1,2-dihydroxyethyl)-4-hydroxy-5-oxo-2,5-dihydrofuran-3-olate
- sodium;(2R)-2-[(1S)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2H-furan-3-olate
Bibliografia____________________________________________________________________
(1) Güler E, Gönülol N, Özyilmaz ÖY, Yücel AÇ. Effect of sodium ascorbate on the bond strength of silorane and methacrylate composites after vital bleaching. Braz Oral Res. 2013 Jul-Aug;27(4):299-304. doi: 10.1590/s1806-83242013000400002.
Abstract. We investigated the effect of sodium ascorbate (SA) on the microtensile bond strengths (MTBSs) of different composites to bovine enamel after vital bleaching with hydrogen peroxide (HP) or carbamide peroxide (CP). Thirty bovine incisors were randomly divided into five groups and treated with no bleaching application (control), 35% HP alone, 35% HP+10% SA for 10 minutes (HP+SA), 16% CP alone, or 16% CP+10% SA for 10 minutes (CP+SA). Specimens were restored with Silorane adhesive and Filtek Silorane composite (designated as S/group) or with Clearfil SE bond and Filtek Supreme XT (designated as F/group). Composite build-up was created on the enamel. Sectioned specimens (n=10 per group; 1 mm2; cross-sectional area) were created and stressed in a universal testing machine at 1 mm/min crosshead speed. The application of 10% SA immediately after bleaching with 16% CP or 35% HP increased the enamel MTBS, regardless of the adhesive/composite resin used. The resulting MTBS values were similar to those of the control groups. Use of 16% CP and 35% HP alone decreased the enamel MTBS, regardless of the adhesive/composite resin used, with F/CP+SA=F/HP+SA=F/CP=S/CP+SA=S/HP+SA=S/C>S/CP=S/HP=F/CP=F/HP (p<0.05). We concluded that the application of SA for 10 minutes immediately after vital bleaching increases the enamel BS for dimethacrylate- and silorane-based composites.
(2) Yoon M, Burrow MF, Wong R, Parashos P. Effect of sodium ascorbate on resin bonding to sodium perborate-bleached dentin. Oper Dent. 2014 Jan-Feb;39(1):98-106. doi: 10.2341/12-516-L.
Abstract. SUMMARY This was an in vitro study to evaluate the effect of sodium ascorbate on the microshear bond strength (MSBS) of resin composite to sodium perborate-bleached dentin. Molar dentin sections were divided into six groups: 1) control, 2) sodium perborate (SP) bleach and immediate bonding, 3) SP and 30 second sodium ascorbate (SA); 4) SP and 1 minute SA; 5) SP and 2 minute SA; and 6) SP and 7 day delay before bonding. They were further divided into two-step self-etching (Clearfil SE Bond) or all-in-one self-etching (Xeno IV) adhesive systems. Resin composite microtubes were bonded according to dentin location-center, pulp horn, and peripheral positions-and an MSBS test was carried out. Failure mode was determined using light microscopy and scanning electron microscopy. There were no significant differences between the treatment types/groups. MSBSs were significantly higher for two-step self-etching adhesive compared with all-in-one self-etching adhesive (p=0.028). For the all-in-one adhesive, MSBSs at the center and pulp horn positions were significantly lower than the peripheral positions (p<0.001). All-in-one groups had significantly more adhesive failures than two-step adhesive groups (p=0.015). The odds of adhesive failure were higher at the pulp horn position than the peripheral position (p=0.004). Sodium perborate bleaching of dentin had no effect on MSBS or mode of failure for either two-step or all-in-one self-etching adhesives; therefore, the effect of sodium ascorbate was negligible. The two-step adhesive groups demonstrated the highest MSBS, and the all-in-one groups, when bonded to center and pulp horn dentin, exhibited the lowest MSBS.
Park JY, Kwon TY, Kim YK. Effective application duration of sodium ascorbate antioxidant in reducing microleakage of bonded composite restoration in intracoronally-bleached teeth. Restor Dent Endod. 2013 Feb;38(1):43-7. doi: 10.5395/rde.2013.38.1.43.
Abstract. Objectives: The aim of this study was to determine an appropriate application duration of sodium ascorbate (SA) antioxidant gel in reducing microleakage of bonded composite restoration in intracoronally-bleached teeth....Results: Group IB showed a significantly higher microleakge than the control group (p = 0.006) and group DB a statistically similar score to the control group (p > 0.999). Although groups S10m, S60m, and S24h exhibited significantly higher scores than group DB (p < 0.05), the microleakage in groups S3d and S7d was statistically similar to that in group DB (p = 0.771, p > 0.999). Conclusions: Application of SA gel for 3 day after nonvital bleaching was effective in reducing microleakage of composite restoration in intracoronally-bleached teeth.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-04-10 19:16:50 | Chemical Risk: |