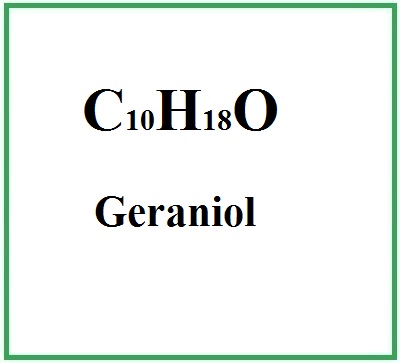
Geraniol is an acyclic monoterpene alcohol.
It is extracted from oils of aromatic plants, such as the herbaceous perennial plant Monarda fistulosa, which belongs to the Lamiaceae family, Cymbopogon martinii var motia, Pelargonium graveolens, and other Cymbopogon species.
Name breakdown and function of the components
- Geraniol - The name is derived from geranium, as geraniol is found in high concentration in the essential oil of this plant. It is a monoterpenic alcohol found in many essential oils.
Description and function of the raw materials used in production
Geranium oil, rose oil, and other essential oils - These oils are the primary natural sources of geraniol. The oil is extracted from plants and flowers that contain the compound.
Summary of the extraction process step by step
- Distillation - Essential oils containing geraniol undergo distillation to separate geraniol from other components.
- Refinement - After distillation, geraniol is further refined to remove impurities.
- Isolation - Finally, geraniol is separated and isolated as a pure compound.
It appears in the form of a light to deep yellow liquid, the scent is intense and the taste is floral rose with a citrus aftertaste.

What it is used for and where
Geraniol has a sweet floral scent and is used in many fragranced products, cosmetics, and as a mosquito repellent. It's also a valuable intermediate in the production of other aromatic compounds.
Medical
Geraniol has demonstrated a broad spectrum of interesting biological activities from a pharmacological point of view, such as antimicrobial properties widely discussed in review papers and studies (1) that have explained the mechanism of penetration into the cell by limiting the growth of the unwanted microorganism. In particular, its effectiveness has been observed against pathogenic microorganisms such as E. coli, S. enterica, Salmonella typhimurium (2) and others.
Although the mechanics of geraniol's molecular activity has not yet been explained with any reasonable degree of certainty, antiproliferative antitumour activity against many human tumours has been observed in both in vitro and in vivo studies by geraniol. This recent study considers that geraniol influences the glycolytic pathway in human cancer cell lines (3).
With regard to anti-inflammatory properties (4), it is believed that geraniol may protect against oxidative changes.
For a complete overview of geraniol's curative potential, see this comprehensive study which lists a large number of them and provides extensive scientific documentation (5). The titles are as follows: Cytotoxic and anti-tumour, Anti-inflammatory and antioxidant, Antimicrobial, Hepatoprotective, Cardioprotective, Anti-diabetic.
It is a component of many essential oils.
Food
Can be used as a fragrance in apples, strawberries and other fruit flavours, cinnamon, ginger and other flavours.
Cosmetics
It is a restricted ingredient as III/78 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: 2,6-Octadien-1-ol, 3,7-dimethyl-, (2E)-
Tonic. This product is used to treat the hair when the scalp is clean or after shampooing. It stimulates hair growth, revitalises the scalp for healthy hair growth and reduces hair loss. It provides a feeling of well-being on skin and hair.
Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.
Other uses
Insect repellent effective against mosquitoes, flies, fire ants, fleas, cockroaches, gnats and ticks
Safety
Geraniol is considered an allergen.
Commercial applications
Perfumery: Due to its rose-like, floral scent, geraniol is frequently employed in perfume-making.
Cosmetic products: Used as a fragrance component in a broad range of products including lotions, creams, bath gels, and cosmetics.
Food flavors: Geraniol is sometimes used as a flavoring in food products.
Insect repellents: Geraniol is also known for its repelling properties and can be incorporated in some insect repellents.
Properties
Monoterpenoid alcohol: Its chemical structure is what provides its aromatic properties.
For more information:
Geraniol studies
Typical optimal commercial product characteristics Geraniol
| Appearance | Clear colorless to pale yellow |
| Density | 0.9±0.1 g/cm3 0.879 g/mL at 20 °C(lit.) |
Boiling Point
| 229.5±0.0°C at 760 mmHg |
Melting Point
| -15 °C |
Flash Point
| 76.7±0.0°C |
Vapour density
| 5.31 (vs air) |
Index of Refraction
| n20/D 1.474(lit.) |
Vapour Pressure
| 0.0±1.0 mmHg at 25°C ~0.2 mm Hg ( 20 °C) |
| Fp | 216 °F |
Solubility in water
| 0.1g/L at 25°C |
| Aroma | Breath of fresh roses |
| pKa | 14.45±0.10 |
Specific Gravity
| 0.878~0.885 (20/4℃) |
Assay,%
| ≥99% |
Water %
| ≤0.4% |
Shelf life
|
|
| Storage | 2-8°C |
| Safety |  |
- Molecular Formula: C10H18O
- Linear Formula (CH3)2C=CHCH2CH2C(CH3)=CHCH2OH
- Molecular Weight 154.253 g/mol
- Exact Mass 154.135757
- CAS: 106-24-1 106-25-2 624-15-7 8007-13-4 68311-14-8 491611-08-6
- UNII: L837108USY
- EC Number: 203-377-1 210-831-2 269-750-6
- FEMA Number: 2507
- DSSTox Substance ID DTXSID8026727 DTXSID7041380
- IUPAC (2E)-3,7-dimethylocta-2,6-dien-1-ol
- InChI=1S/C10H18O/c1-9(2)5-4-6-10(3)7-8-11/h5,7,11H,4,6,8H2,1-3H3/b10-7+
- InChl Key GLZPCOQZEFWAFX-JXMROGBWSA-N
- SMILES CC(=CCCC(=CCO)C)C
- PubChem Substance ID 24849742
- MDL number MFCD00002917
- Beilstein Registry Number 1722456
- JECFA 1223
Synonyms:
- trans-3,7-Dimethyl-2,6-octadien-1-ol
- geraniol, (E)-isomer
- geraniol, (Z)-isomer
- geraniol, 1-(14)C-labeled, (E)-isomer
- geraniol, 2-(14)C-labeled, (E)-isomer
- geraniol, titanium (4+) salt
- Geranyl alcohol
- trans-Geraniol
- (E)-Geraniol
- Geraniol Extra
- 3,7-Dimethyl-2,6-octadien-1-ol
- Geraniol alcohol
- (E)-3,7-Dimethyl-2,6-octadien-1-ol
- beta-Geraniol
- 3,7-Dimethyl-trans-2,6-octadien-1-ol
- 2,6-Octadien-1-ol, 3,7-dimethyl-, (2E)-
- Guaniol
- t-geraniol
- 2E-geraniol
- 2-trans-3,7-Dimethyl-2,6-octadien-1-ol
- (E)-3,7-Dimethylocta-2,6-dien-1-ol
- (2E)-3,7-dimethylocta-2,6-dien-1-ol
- (E)-Nerol
- Lemonol
- 3,7-dimethylocta-2,6-dien-1-ol
- 2,6-Dimethyl-2,6-octadien-8-ol
- 2,6-Octadien-1-ol, 3,7-dimethyl-, trans-
References__________________________________________________________________
(1) Lira M.H.P.D., Andrade Júnior F.P.D., Moraes G.F.Q., Macena G.D.S., Pereira F.D.O., Lima I.O. Antimicrobial activity of geraniol: An integrative review. . J. Essent. Olio Res. 2020; 32:187–197. DOI: 10.1080/10412905.2020.1745697.
Solórzano-Santos F, Miranda-Novales MG. Essential oils from aromatic herbs as antimicrobial agents. Curr Opin Biotechnol. 2012 Apr;23(2):136-41. doi: 10.1016/j.copbio.2011.08.005.
(2) Si W, Gong J, Tsao R, Zhou T, Yu H, Poppe C, Johnson R, Du Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J Appl Microbiol. 2006 Feb;100(2):296-305. doi: 10.1111/j.1365-2672.2005.02789.x.
(3) Crespo R, Rodenak-Kladniew BE, Castro MA, Soberón MV, Lavarías SML. Induction of oxidative stress as a possible mechanism by which geraniol affects the proliferation of human A549 and HepG2 tumor cells. Chem Biol Interact. 2020 Apr 1;320:109029. doi: 10.1016/j.cbi.2020.109029.
(4) Mączka W, Wińska K, Grabarczyk M. One Hundred Faces of Geraniol. Molecules. 2020 Jul 21;25(14):3303. doi: 10.3390/molecules25143303.
Rekha KR, Selvakumar GP, Sethupathy S, Santha K, Sivakamasundari RI. Geraniol ameliorates the motor behavior and neurotrophic factors inadequacy in MPTP-induced mice model of Parkinson's disease. J Mol Neurosci. 2013 Nov;51(3):851-62. doi: 10.1007/s12031-013-0074-9.
(5) Lei Y, Fu P, Jun X, Cheng P. Pharmacological Properties of Geraniol - A Review. Planta Med. 2019 Jan;85(1):48-55. doi: 10.1055/a-0750-6907.
![]() Geraniol
Geraniol