Pentaerythrityl Tetra-Di-T-Butyl Hydroxyhydrocinnamate is a rather complex chemical compound that includes in its structure other chemical compounds such as hydroxycinnamic acid, butanol, and pentaerythrityl :
- Hydroxyhydrocinnamate is a derivative of cinnamic acid, a key intermediate in the metabolism of phenylpropanoids. Cinnamic acid itself is an organic compound with a slightly yellowish colour and a sweet, honey-like aroma. It is used in flavourings, synthetic indigo and some pharmaceutical products.
- T-butyl refers to a functional group (tert-butyl), derived from butane, a type of alkane. The tert-butyl group is distinguished by its bulky nature, which can influence the way the entire compound interacts with others.
- Pentaerythrityl is a quaternary carbon compound derived from the formal condensation of three formaldehyde equivalents and one isobutyraldehyde equivalent.
The synthesis process takes place in different steps:
- The pentaerythritol is first reacted with di-tert-butylphenol in the presence of a catalyst to form the pentaerythrityl tetra-di-tert-butylphenol intermediate.
- This intermediate is then reacted with hydroxycinnamic acid to form the final product, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate.
It typically appears as a white or off-white powder in its pure form. However, when applied as an ingredient in cosmetics or personal care products, the colour of the final product can vary greatly depending on the other ingredients used.

What it is used for and where
It is mainly used to prevent oxidation of oils, fats and fragrances in these products, thus extending their shelf life. It is also known as Butylated Hydroxyhydrocinnamate.
Cosmetics
- Pentaerythrityl Tetra-Di-T-Butyl Hydroxyhydrocinnamate is used in cosmetic products for face and body care as it acts as an antioxidant to prevent the product from degrading when exposed to air and thus oxygen.
- Antioxidant agent. Ingredient that counteracts oxidative stress and prevents cell damage. Free radicals, pathological inflammatory processes, reactive nitrogen species and reactive oxygen species are responsible for the ageing process and many diseases caused by oxidation.
Safety
Given the high molecular weight of this ingredient, dermal penetration is not considered likely. Available toxicity data, together with the low usage concentrations of the ingredient, suggest that systemic toxicity would not be likely if percutaneous absorption occurred.
The Cosmetic Ingredient Review Panel considers this component to be safe in cosmetics in the use practices and concentrations described in this safety assessment (1).
This study examined the migration of Irganox 1010 as antioxidant from high density polyethylene and polypropylene (1).
Commercial Applications
- Antioxidant. Used in cosmetics and skincare products to prevent oxidation and protect the formula from degradation due to exposure to oxygen.
- Skincare products. Due to its antioxidant properties, it's added to lotions, creams, and serums to prolong shelf life and maintain product integrity.
- Cosmetics. Included in products like lipsticks, foundations, and eyeshadows to prevent the oxidation of oils and pigments.
- Hair products. Utilized in shampoos, conditioners, and hair treatments to prevent product deterioration from oxidation.
Medical Applications
- Pharmaceutical Formulations. It may be used as an antioxidant in certain pharmaceutical formulations to protect active ingredients from oxidation and extend stability.
- Dermatology. It could be found in dermatological creams and ointments, where its antioxidant properties might protect the skin and aid in healing.

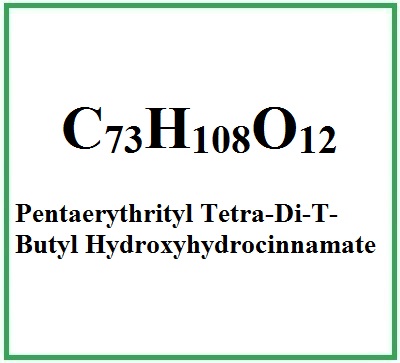
- Molecular Formula C73H108O12
- Linear Formula [HOC6H2[C(CH3)3]2CH2CH2CO2CH2]4C
- Molecular Weight 1177.655 g/mol
- CAS 6683-19-8
- UNII 255PIF62MS
- EC number 229-722-6
- Nikkaji J46.615H
Molar mass: 222.3 g/mol
Formula: C_13H_18O_3
Melting point: 80.11 °C
Boiling point: 397.1 °C
Critical temperature: 872.6 K
Critical pressure: 2.473 MPa
Critical volume: 683.5 cm^3/mol
Molar heat of vaporization: 69.2 kJ/mol
Molar heat of fusion: 20.98 kJ/mol
Molar enthalpy: -558.7 kJ/mol
Molar free energy: -275.8 kJ/mol
Synonyms:
- Pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate)
- 2,2-Bis(((3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoyl)oxy)methyl)propane-1,3-diyl bis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate)
- Irganox 1010
- Irganox 1040
- Tetraalkofen BPE
References______________________________________________________________________
(1) Johnson Jr, W., Bergfeld, W. F., Belsito, D. V., Hill, R. A., Klaassen, C. D., Liebler, D. C., ... & Heldreth, B. (2018). Safety assessment of Pentaerythrityl Tetra-Di-t-Butyl Hydroxyhydrocinnamate as used in cosmetics. International journal of toxicology, 37(3_suppl), 80S-89S.
Abstract. Pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate functions as an antioxidant in cosmetic products and is used at concentrations up to 0.8%. Given the high molecular weight of this ingredient, skin penetration is not likely. The available toxicity data, together with the low ingredient use concentrations, suggest that systemic toxicity would not be likely if percutaneous absorption were to occur. Additionally, the negative human repeated insult patch test data at a concentration of 0.5% were deemed sufficient for evaluating the skin irritation and sensitization potential of pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate over the range of use concentrations in cosmetic products. The Cosmetic Ingredient Review Expert Panel concluded that pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate is safe in cosmetics in the present practices of use and concentration described in this safety assessment.
(2) Lickly TD, Bell CD, Lehr KM. The migration of Irganox 1010 antioxidant from high-density polyethylene and polypropylene into a series of potential fatty-food simulants. Food Addit Contam. 1990 Nov-Dec;7(6):805-14
![]() Pentaerythrityl Tetra-Di-T-Butyl Hydroxyhydrocinnamate
Pentaerythrityl Tetra-Di-T-Butyl Hydroxyhydrocinnamate