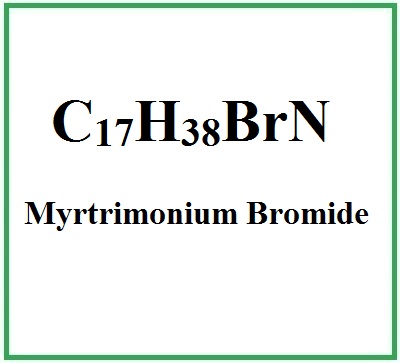
![]() Myrtrimonium Bromide
Myrtrimonium Bromide
Rating : 5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Preservative (1) Antimicrobial (1)9 pts from A_Partyns
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Myrtrimonium Bromide Review Consensus 9 by A_Partyns (12948 pt) | 2023-Aug-07 16:23 |
| Read the full Tiiip | (Send your comment) |
Myrtrimonium bromide is a chemical compound derived from Cetyltrimethylammonium (a surfactant) and is used as a topical antiseptic.
The name defines the structure of the molecule
- Myrtrimonium indicates a specific structure that includes an alkyl chain derived from myristic acid.
- Bromide refers to the bromide anion (Br-) present in the compound, forming a salt with the cationic part of the compound.
Description of raw materials used in production
Myristyl Alcohol: Used as the myristyl group in the molecule.
Ammonia: To provide the quaternary ammonium group.
Bromide: Source of the bromide counterion.
Solvents: Such as water or other suitable solvents for synthesis.
Catalysts: May be used to facilitate the reaction.
Step-by-step industrial chemical synthesis process
- Preparation of Quaternary Ammonium: Myristyl alcohol is reacted with ammonia to form the quaternary ammonium structure.
- Addition of Bromide: A bromide salt, such as sodium bromide or hydrogen bromide, is added to form the bromide counterion.
- Purification: The product is purified using techniques such as crystallization or chromatography.
- Formulation: Myrtrimonium Bromide may be formulated into various products like shampoos, conditioners, or skin-care products for its conditioning and antimicrobial properties.
It appears in the form of a white powder

What it is used for and where
Cosmetics
Widely used in cosmetic products as a preservative, antiseptic.
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Cosmetic biocide. Ingredient with broad-spectrum antibacterial and antifungal activity against the formation of films caused by bacteria.
Antistatic agent.The accumulation of static electricity has a direct influence on products and causes electrostatic adsorption. The antistatic ingredient reduces static build-up and surface resistivity on the surface of the skin and hair.
Commercial applications
Hair Products: Used in shampoos, conditioners, and hair treatments as a conditioning agent to enhance manageability and reduce frizz.
Cosmetic Products: May be used in other skincare products for its emollient properties.
Properties
Conditioning: Helps to improve hair texture, making it softer and more manageable.
Antistatic: Reduces static electricity in the hair, helping to prevent frizz.
Emollient: May act as an emollient, providing a soft feel to the skin.
Most significant studies:
- Myristyltrimethylammonium bromide (MTAB) is a cationic surfactant used to improve biomass harvesting and pigment extraction form microalgae, but the mechanisms underlying its effectiveness are poorly defined. We document the mechanisms for enhanced harvesting and pigment extraction for the cyanobacterium Synechocystis sp. PCC 6803 using measurements from flow cytometer, zeta potential, release of soluble components, and microscopy. Harvesting efficiency increased as the MTAB/Biomass dose increased from 0 to 40%. A low MTAB dose (≤ 8%) mainly brought about coagulation and flocculation, which led to aggregation that improved harvesting, but 40% MTAB had the highest harvesting efficiency, 62%. Adding MTAB above a MTAB/Biomass dose of 8% also increased cell-membrane permeability, which allowed the solvent (ethyl acetate) to pass into the cells and resulted in a large increase in extraction efficiency of pigments: An MTAB/Biomass ratio of 60% for 180 min achieved the highest extraction efficiencies of chlorophyll and carotenoids, 95% and 91%, respectively. Combining harvesting and extraction performances with results from flow cytometry, zeta potential, release of soluble components, and microscopy lead to the following mechanistic understandings. MTAB dose from 8% to 40% solubilized EPS, which lowered the biomass's negative charge, but caused breakup of the large aggregates. An increase of cell permeability also in this stage allowed ethyl acetate to pass into the cells and achieve better pigment extraction. MTAB >40% led to cell lysis and a large increase in soluble organics, but complete cell lysis was not required to achieve the maximum extraction efficiency. The MTAB/Biomass % ratio for optimizing harvest efficiency and pigment extraction lay in the range of 40%-60% (1).
- Dynamin is a GTPase enzyme involved in membrane constriction and fission during endocytosis. Phospholipid binding via its pleckstrin homology domain maximally stimulates dynamin activity. We developed a series of surface-active small-molecule inhibitors, such as myristyl trimethyl ammonium bromide (MiTMAB) and octadecyltrimethyl ammonium bromide (OcTMAB), and we now show MiTMAB targets the dynamin-phospholipid interaction. MiTMAB inhibited dynamin GTPase activity, with a Ki of 940 +/- 25 nM. It potently inhibited receptor-mediated endocytosis (RME) of transferrin or epidermal growth factor (EGF) in a range of cells without blocking EGF binding, receptor number, or autophosphorylation. RME inhibition was rapidly reversed after washout. The rank order of potency for a variety of MiTMAB analogs on RME matched the rank order for dynamin inhibition, suggesting dynamin recruitment to the membrane is a primary cellular target. MiTMAB also inhibited synaptic vesicle endocytosis in rat brain nerve terminals (synaptosomes) without inducing depolarization or morphological defects. Therefore, the drug rapidly and reversibly blocks multiple forms of endocytosis with no acute cellular damage. The unique mechanism of action of MiTMAB provides an important tool to better understand dynamin-mediated membrane trafficking events in a variety of cells (2).
- The aim of the present study was to compare the antimicrobial effect of sodium hypochlorite (NaOCl), 2% chlorhexidine (CHX), a CHX/cetrimide solution (CHX+CTR), octenidine hydrochloride (OCT) and Salvia officinalis plant extract against Enterococcus faecalis. Seventy decoronated single-rooted human teeth were infected and divided into 6 test (n=10) and 2 control groups (n=5) (negative, sterile samples and positive, infected samples). Following irrigants were then applied to test groups: 2.5% NaOCl, 5.25% NaOCl, CHX, CHX+CTR, S. officinalis extract and OCT. The dentin chips were obtained from inner root canal walls and analyzed by counting the number of colony forming units (CFU). The 2.5% NaOCl, 5.25% NaOCl, CHX and OCT groups presented no bacterial growth (CFU=0). S. officinalis and CHX+CTR groups reduced the number of E. faecalis cells but could not eliminate all. OCT may have potential as an endodontic irrigant in treatment of infected root canals (3).
- This study aimed to determine the antimicrobial efficacy of NaOCl, cetrimide, and Glycyrrhiza glabra L. extract against Enterococcus faecalis biofilms on dentine discs. Broth microdilution method was used to determine minimal bactericidal concentrations (MBCs) of the agents. A biofilm susceptibility assay was performed using E. faecalis biofilms grown on dentine discs. Minimal bactericidal concentrations (MBCs) of NaOCl (0.5%), cetrimide (0.015%), and G. glabra L. extract (0.25%) were applied for 1, 3, and 5 min, and the mean viable cell counts were recorded and statistically analyzed. There was no significant difference between cetrimide and NaOCl at 1 min (p>0.05). NaOCl was the most effective agent at 3 and 5 min (p<0.05) while G. glabra L. extract was the least (p<0.05). The MBCs of NaOCl, cetrimide, and G. glabra that eliminated the planktonic E. faecalis did not eradicate the biofilms grown on dentin discs (4).

- Molecular Formula C17H38BrN
- Molecular Weight 336.402 g/mol
- CAS 1119-97-7 8044-71-1 114568-24-0
- UNII: 8483H94W1E
- EC Number: 214-291-9 617-073-5
- Cetrimide
- 1-hexadecyltrimethylammonium chloride
- cetriminium
- cetrimonium
- cetrimonium bromide
- cetrimonium chloride
- cetrimonium hydroxide
- cetrimonium iodide
- cetrimonium methyl sulfate
- cetrimonium monosulfate
- cetyltrimethylammonium bromide
- cetyltrimethylammonium chloride
- Octylsulfonate, Hexadecyltrimethylammonium
- hexadecyl trimethyl ammonium bromide
- hexadecyl(trimethyl)azanium
- hexadecyltrimethylammonium bromide
- hexadecyltrimethylammonium octylsulfonate
- Myristyltrimethylammonium bromide
- Tetradecyltrimethylammonium bromide
- Tetradonium bromide
- Morpan T
- Alkyltrimethylammonium bromide
- CHEBI:3565
- Myristyltrimethylaminium bromide
- Myristyl trimethyl ammonium bromide
- N-Tetradecyltrimethylammonium bromide
- trimethyltetradecylamine, bromide
- 1-Tetradecanaminium, N,N,N-trimethyl-, bromide (1:1)
- trimethyl(tetradecyl)azanium;bromide
- CHEMBL113150
- Trimethyltetradecyl Ammonium Bromide
- Quaternium 13
- Trimethyl(tetradecyl)ammonium bromide
- 1-Tetradecanaminium, N,N,N-trimethyl-, bromide
- Trimethyltetradecylammonium bromide
- Tetradonium bromide [INN]
- N,N,N-Trimethyl-1-tetradecanaminium bromide
- trimethyl(tetradecyl)azanium bromide
- Ammonium, trimethyltetradecyl-, bromide
- Cetrimonium Methosulfate
References__________________________________________________________________
(1) Zhou Y, Lai YS, Eustance E, Straka L, Zhou C, Xia S, Rittmann BE. How myristyltrimethylammonium bromide enhances biomass harvesting and pigments extraction from Synechocystis sp. PCC 6803. Water Res. 2017 Dec 1;126:189-196. doi: 10.1016/j.watres.2017.09.036. Epub 2017 Sep 21
(2) Quan A, McGeachie AB, Keating DJ, van Dam EM, Rusak J, Chau N, Malladi CS, Chen C, McCluskey A, Cousin MA, Robinson PJ. Myristyl trimethyl ammonium bromide and octadecyl trimethyl ammonium bromide are surface-active small molecule dynamin inhibitors that block endocytosis mediated by dynamin I or dynamin II. Mol Pharmacol. 2007 Dec;72(6):1425-39. Epub 2007 Aug 16
(3) Guneser MB, Akbulut MB, Eldeniz AU. Antibacterial effect of chlorhexidine-cetrimide combination, Salvia officinalis plant extract and octenidine in comparison with conventional endodontic irrigants. Dent Mater J. 2016;35(5):736-741.
(4) Güldas HE, Kececi AD, Cetin ES, Ozturk T, Kaya BÜ. Evaluation of antimicrobial efficacy of cetrimide and Glycyrrhiza glabra L. extract against Enterococcus faecalis biofilm grown on dentin discs in comparison with NaOCl. Dent Mater J. 2016 Oct 1;35(5):721-727 28.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2019-05-13 21:06:43 | Chemical Risk: |