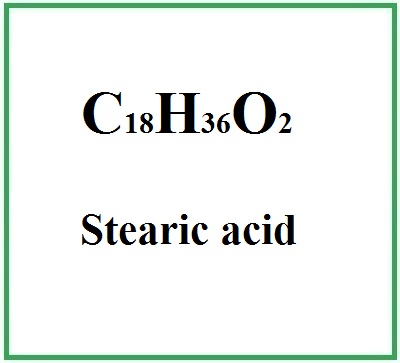
![]() Stearic acid
Stearic acid
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Anti-inflammatory (1)Cons:
Probable animal origin (1)9 pts from A_Partyns
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Stearic Acid studies" about Stearic acid by A_Partyns (12948 pt) | 2022-Oct-04 19:13 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Kritchevsky D. Stearic acid metabolism and atherogenesis: history. Am J Clin Nutr. 1994 Dec;60(6 Suppl):997S-1001S. doi: 10.1093/ajcn/60.6.997S.
Abstract. Studies conducted in dogs, rats, and hamsters show that stearic acid or stearic acid-rich glycerides are absorbed less efficiently than are lauric, myristic, and palmitic acids or their triglycerides. This observation may explain in part why stearic acid is less cholesterolemic than saturated fatty acids of shorter chain length. In rabbits, cocoa butter or other fats rich in stearic acid are less atherogenic than other saturated fatty acids. This finding is true for both cholesterol-containing and cholesterol-free diets.
Vanderveen JE. Regulatory history for stearic acid. Am J Clin Nutr. 1994 Dec;60(6 Suppl):983S-985S. doi: 10.1093/ajcn/60.6.983S.
Abstract. Before 1974 the only regulations involving stearic acid were for its use as a food additive. In 1974 the regulation for fat, fatty acid, and cholesterol contents was finalized; this regulation defined saturated fatty acid as the sum of lauric, myristic, palmitic, and stearic acids. Because the labeling of saturated fatty acid was voluntary except when a claim was made for fat content, the inclusion of stearic acid in that definition had little impact on foods high in fatty acids. Under the requirements of the Nutrition Labeling and Education Act (NLEA) of 1990, the definition of a saturated fatty acid gained major significance, with ties to mandatory nutrition labeling, nutrient content claims, and health claims. It was requested that stearic acid be dropped from the definition of a saturated fatty acid because it did not raise blood cholesterol concentrations. Scientific data demonstrating the lack of involvement of stearic acid consumption in negative health effects are needed.
Habib NA, Wood CB, Apostolov K, Barker W, Hershman MJ, Aslam M, Heinemann D, Fermor B, Williamson RC, Jenkins WE, et al. Stearic acid and carcinogenesis. Br J Cancer. 1987 Oct;56(4):455-8. doi: 10.1038/bjc.1987.223.
Abstract. Decreased membrane rigidity is one of the characteristics of malignant cells, resulting in part from the desaturation of stearic acid into oleic acid. In this study we investigated the influence of stearic acid on tumour cell inhibition in vitro and tumour development in vivo. Stearic acid inhibited the colony-forming ability of 4 out of 5 rat and two human tumour continuous cell lines in vitro. In contrast, the colony-forming ability of rat fibroblasts was not inhibited and that of human foetal lung fibroblasts was inhibited at a higher dose than that required to inhibit human tumour cell lines. Using a model of rat mammary carcinoma induced by nitroso-methyl urea (NMU) the subcutaneous injection of stearic acid at weekly intervals prevented tumour development in 5 to 10 rats. Using iodostearic acid twice weekly, 11 of 19 rats were alive and tumour free at week 22 whilst all of 14 animals injected with NMU alone had died of tumour by the 16th week. The ratio of stearic to oleic acids in erythrocyte membranes was significantly reduced in the tumour-bearing rats, but was normal in tumour-free animals treated with stearic or iodostearic acid. These preliminary data indicate that stearic acid inhibits tumour development in rats.
van Rooijen MA, Plat J, Blom WAM, Zock PL, Mensink RP. Dietary stearic acid and palmitic acid do not differently affect ABCA1-mediated cholesterol efflux capacity in healthy men and postmenopausal women: A randomized controlled trial. Clin Nutr. 2021 Mar;40(3):804-811. doi: 10.1016/j.clnu.2020.08.016.
Abstract. ...Objective: We examined effects of exchanging dietary palmitic acid for stearic acid on ATP-binding cassette transporter A1 (ABCA1)-mediated CEC, and other conventional and emerging cardiometabolic risk makers....Copyright © 2020 Elsevier Ltd and European Society for Clinical Nutrition and Metabolism.
Kris-Etherton PM, Griel AE, Psota TL, Gebauer SK, Zhang J, Etherton TD. Dietary stearic acid and risk of cardiovascular disease: intake, sources, digestion, and absorption. Lipids. 2005 Dec;40(12):1193-200. doi: 10.1007/s11745-005-1485-y.
Abstract. Individual FA have diverse biological effects, some of which affect the risk of cardiovascular disease (CVD). In the context of food-based dietary guidance designed to reduce CVD risk, fat and FA recommendations focus on reducing saturated FA (SFA) and trans FA (TFA), and ensuring an adequate intake of unsaturated FA. Because stearic acid shares many physical properties with the other long-chain SFA but has different physiological effects, it is being evaluated as a substitute for TFA in food manufacturing. For stearic acid to become the primary replacement for TFA, it is essential that its physical properties and biological effects be well understood.
van Rooijen MA, Plat J, Zock PL, Blom WAM, Mensink RP. Effects of two consecutive mixed meals high in palmitic acid or stearic acid on 8-h postprandial lipemia and glycemia in healthy-weight and overweight men and postmenopausal women: a randomized controlled trial. Eur J Nutr. 2021 Oct;60(7):3659-3667. doi: 10.1007/s00394-021-02530-2.
Abstract. Purpose: Palmitic and stearic acids have different effects on fasting serum lipoproteins. However, the effects on postprandial lipemia and glycemia are less clear. Also, the effects of a second meal may differ from those of the first meal. Therefore, we studied the effects of two consecutive mixed meals high in palmitic acid- or stearic acid-rich fat blends on postprandial lipemia and glycemia....Conclusion: Consumption of the stearic acid-rich meals lowered postprandial lipemia as compared with palmitic acid. After the second stearic acid-rich meal, concentrations of C-peptide peaked earlier and those of NEFA decreased more. Clinical trial registry This trial was registered at clinicaltrials.gov as NCT02835651 on July 18, 2016. © 2021. The Author(s).
Tsuchiya A, Kanno T, Nishizaki T. Stearic acid serves as a potent inhibitor of protein tyrosine phosphatase 1B. Cell Physiol Biochem. 2013;32(5):1451-9. doi: 10.1159/000356582.
Abstract. Background/aims: Free fatty acids (FFAs) are implicated in diverse signal transduction pathways. The present study investigated the effects of the saturated FFA stearic acid on protein tyrosine phosphatase 1B (PTP1B) activity, Akt activity, and glucose uptake into cells relevant to insulin signal....Conclusion: The results of the present study indicate that stearic acid serves as a potent PTP1B inhibitor, possibly causing an enhancement in the insulin receptor signaling to stimulate glucose uptake into adipocytes. © 2013 S. Karger AG, Basel.
Cobb TK. Effects of dietary stearic acid on plasma cholesterol levels. South Med J. 1992 Jan;85(1):25-7. doi: 10.1097/00007611-199201000-00007.
Abstract. Dietary advice based on saturated versus unsaturated fatty acids for the purpose of controlling the plasma cholesterol level has for many years plagued the American public with unpalatable diets that are difficult to adhere to. Stearic acid, an 18-carbon saturated fatty acid, does not share the hypercholesterolemic effect of other saturated fatty acids and may be the key to more palatable diets for the cholesterol-conscious dieter in the future.
Spigoni V, Fantuzzi F, Fontana A, Cito M, Derlindati E, Zavaroni I, Cnop M, Bonadonna RC, Dei Cas A. Stearic acid at physiologic concentrations induces in vitro lipotoxicity in circulating angiogenic cells. Atherosclerosis. 2017 Oct;265:162-171. doi: 10.1016/j.atherosclerosis.2017.09.004.
Abstract. Background and aims: Saturated free fatty acids (SFAs) can induce lipotoxicity in different cells. No studies have investigated the effects of SFA in circulating angiogenic cells (CACs), which play a key role in endothelial repair processes. The aim of the study was to assess the effects of SFAs, specifically stearic acid (SA), on viability and function of CACs and to investigate potential underlying molecular mechanisms....Conclusions: In humans, both function and viability of CACs are exquisitely vulnerable to physiologic concentrations of stearate; lipotoxic impairment of endothelial repair processes may be implicated in vascular damage caused by SFAs. Copyright © 2017 Elsevier B.V.
Berry SE. Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: an overview and implications for cardiovascular disease. Nutr Res Rev. 2009 Jun;22(1):3-17. doi: 10.1017/S0954422409369267.
Abstract. The position of fatty acids in the TAG molecule (sn-1, sn-2 and sn-3) determines the physical properties of the fat, which affects its absorption, metabolism and distribution into tissues, which may have implications for the risk of CHD. The TAG structure of fats can be manipulated by the process of interesterification, which is of increasing commercial importance, as it can be used to change the physical characteristics of a fat without the generation of trans-fatty acids. Interesterified fats rich in long-chain SFA are commercially important, but few studies have investigated their health effects. Evidence from animal and human infant studies suggests that TAG structure and interesterification affect digestibility, atherogenicity and fasting lipid levels, with fats containing palmitic and stearic acid in the sn-2 position being better digested and considered to be more atherogenic. However, chronic studies in human adults suggest that TAG structure has no effect on digestibility or fasting lipids. The postprandial effects of fats with differing TAG structure are better characterised but the evidence is inconclusive; it is probable that differences in the physical characteristics of fats resulting from interesterification and changes in TAG structure are key determinants of the level of postprandial lipaemia, rather than the position of fatty acids in the TAG. The present review gives an overview of TAG structure and interesterified palmitic and stearic acid-rich fats, their physical properties and their acute and chronic effects in human adults in relation to CHD.
Aro A, Jauhiainen M, Partanen R, Salminen I, Mutanen M. Stearic acid, trans fatty acids, and dairy fat: effects on serum and lipoprotein lipids, apolipoproteins, lipoprotein(a), and lipid transfer proteins in healthy subjects. Am J Clin Nutr. 1997 May;65(5):1419-26. doi: 10.1093/ajcn/65.5.1419.
Abstract. To compare the effects on serum lipoproteins of stearic acid, trans fatty acids, and dairy fat, 80 healthy subjects consumed a dairy fat-based (baseline) diet for 5 wk, then an experimental diet high in either trans fatty acids (8.7% of energy; n = 40) or stearic acid (9.3% of energy; n = 40) for another 5 wk. All diets provided 32.2-33.9% of energy as fat, 14.6-15.8% as saturated plus trans fatty acids, 11.4-12.5% as cis-monounsaturated fatty acids, 2.9-3.5% as polyunsaturated fatty acids, and 200-221 mg cholesterol/10 MJ. Compared with the dairy fat diet, stearic acid and trans fatty acids decreased serum total cholesterol concentrations similarly (by 13% and 12%, respectively, P < 0.001) but the trans fatty acid diet decreased HDL cholesterol (17%) and apolipoprotein (apo) A-I (15%) significantly more than did the stearic acid diet (11% and 12%, respectively). Stearic acid but not trans fatty acids reduced concentrations of LDL cholesterol and apo B significantly (P < 0.001). The trans fatty acid diet increased the ratio of LDL to HDL cholesterol (19%) and of apo B to apo A-I (16%) more than did the dairy fat diet (P < 0.001) but the stearic acid diet had no effect. Lipoprotein(a) concentrations increased with both experimental diets, significantly more with trans fatty acids (30%) than with stearic acid (10%). In conclusion, high amounts of trans fatty acids had more adverse effects on lipoproteins than did equal amounts of stearic acid and dairy fat. Stearic acid reduced LDL cholesterol, did not affect the ratio of LDL to HDL cholesterol, and increased lipoprotein(a), although to a lesser extent than did trans fatty acids. Dietary fats low in both saturated fatty acids and trans fatty acids should be favored.
Tuomasjukka S, Viitanen M, Kallio H. Stearic acid is well absorbed from short- and long-acyl-chain triacylglycerol in an acute test meal. Eur J Clin Nutr. 2007 Dec;61(12):1352-8. doi: 10.1038/sj.ejcn.1602658.
Abstract. Objective: Absorption of stearic acid from natural oils has been shown to be efficient, but it is claimed to be lower from short- and long-acyl-chain triacylyglycerol molecules (Salatrim). The aim was to measure the apparent absorption of stearic acid from Salatrim fat in an acute test meal. Design: Double-blind crossover study. Conclusions: The apparent absorption of stearic acid does not differ from its absorption from natural fats. The status of Salatrim as a low-energy fat substitute needs to be re-evaluated.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Stearic acid Review Consensus 9 by A_Partyns (12948 pt) | 2022-Oct-04 21:12 |
| Read the full Tiiip | (Send your comment) |
Stearic acid is a long-chain (18:0) dietary saturated fatty acid. It occurs naturally in animal and vegetable fats. For example, in soya beans where its content is typically between 2% and 5% of total fatty acids. It is produced by hydrolysis of oil
It appears as a white powder insoluble in water, easily soluble in hot ethanol, slightly soluble in benzene and carbon disulphide. Tasteless.

What it is used for and where
Food
Recent investigations specifically comparing stearic acid with other fatty acids in human studies have confirmed that stearic acid is not hypercholesterolaemic. Stearic acid has been shown not to increase low-density lipoprotein cholesterol compared to oleic acid, which is known to be neutral in its effects on cholesterol concentrations. In contrast, palmitic acid, another long-chain saturated fatty acid, markedly increases cholesterol concentrations. For this reason, fats rich in stearic acid could be used instead of those high in palmitic acid in cholesterolaemic diets (1).
Medical
Stearic acid has been shown to possess anti-inflammatory potential. In this study, it was evaluated whether stearic acid has protective effects against cholestasis-related liver damage and indeed its hepatoprotective role was found to be associated with anti-inflammatory potential (2).
Stearic acid can protect cortical neurons from oxidative stress by increasing internal antioxidant enzymes (3).
Chemical synthesis and characterisation of a lipophilic ester conjugate, propofol stearate, and evaluation of its antitumour efficacy on human breast cancer cell lines MDA-MB-361, MCF-7 and MDA-MB-231. Exogenous stearic acid applied as an ester derivative inhibits the growth of human breast cancer cells and shows a beneficial role in the treatment of breast cancer in vitro (4).
Skin disorders are often treated with creams containing various active substances that also contain emulsifiers, surfactant ingredients used to stabilise the emulsion. Emulsifiers are potential irritants and in the present study, the influence of stearic acid did not show any differences with regard to blood flow in the skin. Furthermore, stearic acid as emulsifier unexpectedly reduced transepidermal water loss (TEWL). These results point to the possibility of absorption of stearic acid as emulsifier into the lipid bilayer, which increases TEWL in normal skin and decreases TEWL in damaged skin (6).
Used to prepare suppositories, ointments, etc.
Cosmetics
Stearic acid is incorporated into skin care products as a non-ionic emulsifier, solubiliser, and lubricant. Emulsifiers have the property of reducing interfacial tension and also directly influence the stability, sensory properties and surface tension of sunscreens by modulating their filmometric performance.
Pharmaceuticals
Coating agent for tablets.
Other uses
Raw material for lubricants, plasticisers, PVC stabiliser, vulcanising agent for natural rubber, synthetic rubber (except butyl rubber) and latex.
For more information:
Typical commercial product characteristics Stearic Acid
| Appearance | White powder |
| Boiling Point | 361°C |
| Melting Point | 67-72°C |
| Flash Point | >230 °F |
| Density | 0.84 |
| Vapor Pressure | 1 mm Hg 173.7°C |
| Refraction Index | 1.4299 |
| Water Solubility | 0.1-1 g/100 mL/ 23ºC |
| PSA | 37.30000 |
| LogP | 6.33250 |
| Saponification value | 195-211 mg KOH /g |
| Acid value | 195-211g mg KOH /g |
| Iodine | ≤0.6 |
| Water content | ≤0.1 |
| C16 | 4 ~ 59% |
| C18 | 37 ~ 96% |
| Shelf life | 2 years |
| Chemical risk |  |




- Molecular Formula C18H36O2
- Linear Formula CH3(CH2)16COOH
- Molecular Weight 284,4772
- Exact Mass 284.27200
- CAS 57-11-4
- UNII 4ELV7Z65AP
- EC Number 266-928-5 200-313-4
- DSSTox Substance ID DTXSID8021642
- IUPAC octadecanoic acid
- InChl=1S/C18H36O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h2-17H2,1H3,(H,19,20)
- InChl Key QIQXTHQIDYTFRH-UHFFFAOYSA-N
- SMILES CCCCCCCCCCCCCCCCCC(=O)O
- MDL number MFCD00002752
- PubChem Substance ID 24899616
- ChEBI 28842
- RTECS WI2800000
- Beilstein 608585
- NCI C68426
- ICSC 0568
- NSC 25956
- NSC 261168
- FEMA 3035
- JECFA 116
- RXCUI 1310551
- NACRES NA.25
Synonyms
- Octadecanoic acid
- Cetylacetic acid
- 1-Heptadecanecarboxylic acid
- Pearl stearic
- n-Octadecanoic acid
- Stearophanic acid
References______________________________________________________________
(1) Grundy SM. Influence of stearic acid on cholesterol metabolism relative to other long-chain fatty acids. Am J Clin Nutr. 1994 Dec;60(6 Suppl):986S-990S. doi: 10.1093/ajcn/60.6.986S.
(2) Pan PH, Lin SY, Ou YC, Chen WY, Chuang YH, Yen YJ, Liao SL, Raung SL, Chen CJ. Stearic acid attenuates cholestasis-induced liver injury. Biochem Biophys Res Commun. 2010 Jan 15;391(3):1537-42. doi: 10.1016/j.bbrc.2009.12.119.
(3) Wang ZJ, Liang CL, Li GM, Yu CY, Yin M. Stearic acid protects primary cultured cortical neurons against oxidative stress. Acta Pharmacol Sin. 2007 Mar;28(3):315-26. doi: 10.1111/j.1745-7254.2007.00512.x.
(4) Khan AA, Alanazi AM, Jabeen M, Chauhan A, Abdelhameed AS. Design, synthesis and in vitro anticancer evaluation of a stearic acid-based ester conjugate. Anticancer Res. 2013 Jun;33(6):2517-24.
(6) Bárány E, Lindberg M, Lodén M. Unexpected skin barrier influence from nonionic emulsifiers. Int J Pharm. 2000 Feb 15;195(1-2):189-95. doi: 10.1016/s0378-5173(99)00388-9.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2015-11-17 19:10:09 | Chemical Risk: |