![]() Quinine monochloride dihydrate
Quinine monochloride dihydrate
Rating : 5.5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antioxidant (1)Cons:
Possible risk. Click on ingredient (1)8 pts from Nat45
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Quinine studies" about Quinine monochloride dihydrate Review Consensus 8 by A_Partyns (12948 pt) | 2019-Jun-03 09:32 |
| Read the full Tiiip | (Send your comment) |
Analytics of Quinine and its Derivatives.
Kluska M, Marciniuk-Kluska A, Prukała D, Prukała W
Crit Rev Anal Chem. 2016;46(2):139-45. doi: 10.1080/10408347.2014.996700.
Artesunate versus Quinine - keeping our options open.
Frosch AEP.
Clin Infect Dis. 2019 May 2. pii: ciz335. doi: 10.1093/cid/ciz335.
Protective effects of pyrroloquinoline quinine against oxidative stress-induced cellular senescence and inflammation in human renal tubular epithelial cells via Keap1/Nrf2 signaling pathway.
Wang Z, Han N, Zhao K, Li Y, Chi Y, Wang B.
Int Immunopharmacol. 2019 Jul;72:445-453. doi: 10.1016/j.intimp.2019.04.040.
Synthesis and systemic toxicity assessment of quinine-triazole scaffold with antiprotozoal potency.
Sahu A, Agrawal RK, Pandey R.
Bioorg Chem. 2019 Apr 20;88:102939. doi: 10.1016/j.bioorg.2019.102939.
Shape adaptation of quinine in cyclodextrin cavities: NMR studies.
Wójcik J, Ejchart A, Nowakowski M.
Phys Chem Chem Phys. 2019 Mar 27;21(13):6925-6934. doi: 10.1039/c9cp00590k.
Pyrroloquinoline quinine protects HK-2 cells against high glucose-induced oxidative stress and apoptosis through Sirt3 and PI3K/Akt/FoxO3a signaling pathway.
Wang Z, Li Y, Wang Y, Zhao K, Chi Y, Wang B.
Biochem Biophys Res Commun. 2019 Jan 8;508(2):398-404. doi: 10.1016/j.bbrc.2018.11.140. Epub 2018 Nov 28.
Effects of Quinine, Quinidine and Chloroquine on Human Muscle Nicotinic Acetylcholine Receptors.
Gisselmann G, Alisch D, Welbers-Joop B, Hatt H.
Front Pharmacol. 2018 Nov 20;9:1339. doi: 10.3389/fphar.2018.01339. eCollection 2018.
High-level artemisinin-resistance with quinine co-resistance emerges in P. falciparum malaria under in vivo artesunate pressure.
Tyagi RK, Gleeson PJ, Arnold L, Tahar R, Prieur E, Decosterd L, Pérignon JL, Olliaro P, Druilhe P.
BMC Med. 2018 Oct 1;16(1):181. doi: 10.1186/s12916-018-1156-x.
Use of hyperbaric oxygen therapy in quinine-associated visual disturbances.
Laes JR, Hendriksen S, Cole JB.
Undersea Hyperb Med. 2018 Jul-Aug;45(4):457-461.
fMRI-Based Brain Responses to Quinine and Sucrose Gustatory Stimulation for Nutrition Research in the Minipig Model: A Proof-of-Concept Study.
Coquery N, Meurice P, Janvier R, Bobillier E, Quellec S, Fu M, Roura E, Saint-Jalmes H, Val-Laillet D.
Front Behav Neurosci. 2018 Jul 24;12:151. doi: 10.3389/fnbeh.2018.00151. eCollection 2018.
A Case Report of Nephrotic Syndrome While Undergoing Quinine Therapy.
Albrecht B, Giebel S, McCarron M, Prasad B.
Cureus. 2018 Mar 7;10(3):e2283. doi: 10.7759/cureus.2283.
The Role of Quinine-Responsive Taste Receptor Family 2 in Airway Immune Defense and Chronic Rhinosinusitis.
Workman AD, Maina IW, Brooks SG, Kohanski MA, Cowart BJ, Mansfield C, Kennedy DW, Palmer JN, Adappa ND, Reed DR, Lee RJ, Cohen NA.
Front Immunol. 2018 Mar 28;9:624. doi: 10.3389/fimmu.2018.00624. eCollection 2018.
Intragastric quinine administration decreases hedonic eating in healthy women through peptide-mediated gut-brain signaling mechanisms.
Iven J, Biesiekierski JR, Zhao D, Deloose E, O'Daly OG, Depoortere I, Tack J, Van Oudenhove L.
Nutr Neurosci. 2018 Apr 2:1-13. doi: 10.1080/1028415X.2018.1457841.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Quinine in bitter drinks" about Quinine monochloride dihydrate Review Consensus 8 by A_Partyns (12948 pt) | 2023-Jul-18 18:02 |
| Read the full Tiiip | (Send your comment) |
The best study I have yet met on the indications and contraindications of quinine in bitter drinks, is undoubtedly the one published in 2008 by BfR which is the German Federal Institute for Risk Assessment and was established as a federal authority under the jurisdiction of the Federal Ministry of Food and German agriculture (BmEL). Here is the first part. The study is very long, about 16 pages and contains all the bibliographical references:
Quinine is a bitter-tasting, crystalline white powder. It is obtained from the bark of the cinchona tree and belongs to the group of alkaloids. In medicine quinine is used to treat malaria and nocturnal leg cramps. In the food sector, quinine is used as a flavouring mainly in beverages like bitter lemon and tonic water.

When larger amounts of quinine are consumed, it can constitute a health problem for some consumer groups. BfR sees risks in particular for quinine intakes during pregnancy. For instance, a newborn baby, whose mother had drunk more than 1 litre tonic water a day in the weeks up to its birth, suffered health disorders. Based on existing regulations in the medicinal product sector, BfR, therefore, advises pregnant women against drinking quininecontaining beverages on precautionary grounds. People who have been advised against taking quinine, cinchona bark or their preparations by their doctors because of their clinical pictures should not consume any quinine-containing soft drinks either. This applies, for instance, to people who suffer from tinnitus, pre-existing damage to the optic nerve, haemolytic anaemia or who are hypersensitive to quinine or cinchona alkaloids. Patients with cardiac arrhythmia and people who take medicine that interacts with quinine, should only drink quinine-containing soft drinks after consulting their doctors. This applies in particular to medications which inhibit blood coagulation. At higher levels of tonic water consumption, it may be necessary to reduce their therapeutic dose.
Already today quinine must be mentioned by name in the list of ingredients of quininecontaining products. BfR also believes that there is a need for information which attracts the attention more particularly of pregnant women and other risk groups to possible health impairments. Motor vehicle drivers should be informed that larger amounts of quininecontaining bitter beverages can cause visual disturbances. BfR recommends raising awareness about the possible health risks from quinine to consumers. Specific information should be provided about the symptoms of quinine hypersensitivity and cinchonism (typical adverse reactions to quinine). Consumers should be advised to immediately stop their quinine intake if these symptoms occur, and to consult a doctor.
BfR recommends that the health assessment of quinine by the Scientific Committee on Food from 1988 should be updated.
BfR is of the opinion that the problems of quinine-containing bitter soft drinks underline the importance of the systematic recording of adverse reactions that occur in conjunction with the consumption of foods. The Institute, therefore, explicitly supports the setting up of a central reporting office (1).
References________________________________________________
(1) Quinine-containing beverages may cause health problems
Updated BfR Health Assessment No 020/2008, 17 February 2005
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Quinine monochloride dihydrate Review Consensus 8 by Nat45 (5724 pt) | 2023-Jul-18 17:57 |
| Read the full Tiiip | (Send your comment) |
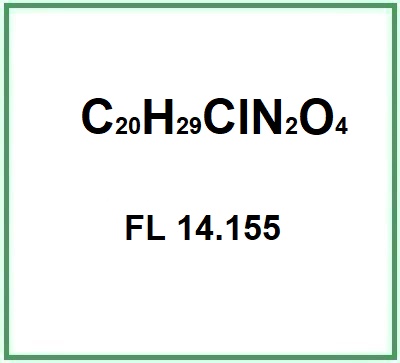
Quinine monochloride dihydrate (Quinine monohydrochloride dihydrate) is a salt obtained chemically from quinine. Quinine is an alkaloid from the bark of the China tree, family Rubiaceae. Three salts are obtained from quinine: FL 14,011, FL 14,152 and FL 14,155, and the latter is what is called Quinine monochloride dihydrate. The specific properties of Quinine monochloride dihydrate, such as solubility and stability, may vary depending on storage and usage conditions.
Quinine occurs naturally in the bark of the cinchona tree and was the first effective western treatment for malaria.
The name defines the structure of the molecule:
- Quinine is the natural compound found in the bark of the cinchona tree. Quinine has been used for centuries as a treatment for malaria because of its ability to kill disease-causing parasites.
- Monochloride refers to a compound containing a chlorine atom. In the context of quinine monochlorine, it means that a chlorine atom is attached to the quinine molecule.
- Dihydrate refers to a compound that has two water molecules (H2O) associated with each formula unit of the compound. In the context of quinine monochlorine dihydrate, it means that two water molecules are associated with each molecule of quinine monochlorine.
The raw materials for the production of quinine monochloride dihydrate are:
- Quinine - This alkaloid is extracted from the bark of the Cinchona tree. Quinine has antimalarial properties and is used to produce various drugs.
- Hydrochloric acid - This is a strong acid used to convert quinine into quinine hydrochloride. The reaction with hydrochloric acid neutralizes the base of quinine, forming the hydrochloride salt.
- Water - Water is used to create the dihydrate form of quinine hydrochloride. Two water molecules bind to the quinine hydrochloride to form the dihydrate.
The synthesis process takes place in several stages:
- Extraction of quinine from the bark of the Cinchona tree.
- Reaction. The extracted quinine is reacted with hydrochloric acid to form quinine hydrochloride.
- Crystallization. Quinine hydrochloride is crystallized in the presence of water to form monochlorinated quinine dihydrate.
Monochlorinated quinine dihydrate is a solid at standard temperature and pressure (STP), with a melting point of 115.5 ºC. It is typically found in powder form. The color may vary depending on its purity and the specific production process, but it is typically white or off-white.

What it is used for and where
Food
It is used in drinks and some foods to impart a bitter taste.
Contraindications - studies
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common human enzyme defect that often appears in neonatal jaundice and/or haemolytic anaemia. G6PD haemolytic events are linked to exposure to a pro-oxidant agent. Here we report three cases of initial G6PD crises in breastfed infants with maternal consumption of a tonic drink containing quinine. Quinine was found in the breast milk of one of the mothers after consuming tonic water. The amount of quinine that is transmitted through breast milk appears to be sufficient to induce G6PD seizures in breastfed infants. Therefore, it is recommended to avoid consumption of quinine-containing drinks during breastfeeding in populations with a high prevalence of G6PD deficiency.
What is known:
- G6PD haemolytic events are linked to exposure to a pro-oxidant agent.
- The ingestion of fava beans by a mother who was breastfeeding induced a neonatal G6PD crisis.
What's new:
- maternal consumption of a tonic drink containing quinine appears to be sufficient to induce G6PD seizures in breastfed infants.
- Maternal consumption of quinine-containing drinks during breastfeeding should be avoided in populations with a high prevalence of G6PD deficiency (1).
Quinine is a common cause of drug-induced thrombocytopenia and the most common cause of drug-induced thrombotic microangiopathy. Other quinine-induced systemic disorders have been described. In order to understand the full clinical spectrum of adverse reactions to quinine, the author of this study consulted 11 databases that provided sufficient data to allow evaluation of levels of evidence supporting a causal association with quinine. Three reviewers independently determined levels of evidence, including both immune-mediated and toxic adverse reactions. The main focus of this review was on acute, immune-mediated reactions. The source of quinine exposure, organ systems involved, severity of adverse reactions and patient outcomes were documented. One hundred and fourteen articles described 142 patients with definite or probable evidence of a causal association of quinine with acute, immune-mediated reactions. One hundred and nine (72%) reactions were caused by quinine pills; 28 (20%) by quinine-containing beverages; 12 (8%) by five other types of exposure.Quinine, even with minimal exposure from common beverages, can cause severe adverse reactions involving multiple organ systems. In patients with acute, multisystem disorders of unknown origin, an adverse reaction to quinine must be considered (2).
European legislation has dealt with this substance (3) in order to assess its effects on human health and, in the opinion of the Scientific Committee on Food (SCF) of 19 February 1988, no objections were raised, from a toxicological point of view, to the continued use of quinine in bitter drinks at current levels (up to a maximum of 100 mg/l). While not contesting this assessment, the Authority recommends a review of the toxicological database on quinine (4). Pending the re-evaluation of quinine, the use of three quinine salts (FL 14.011, FL 14.152 and FL 14.155) should be restricted to non-alcoholic and alcoholic beverages.

- Molecular Formula C20H29ClN2O4
- Molecular Weight 396.91
- CAS 6119-47-7
- EC number 231-437-7
- UNII 711S8Y0T33
Synonyms :
- 60-93-5 (.2HCl)
- AKOS025310495
- BIQ1204
- CHEMBL3706390
- Chinini hydrochloridum
- Cinchonan-9-ol, 6'-methoxy-, monohydrochloride, dihydrate, (8a,9R)-
- CS-2539
- DTXSID2047694
- FL 14.155
- HY-B0433A
- KS-000010VZ
- LS40029
- MFCD00078498
- MolPort-003-926-508
- Quinine HCl Dihydrate
- QUININE HYDROCHLORIDE DIHYDRATE
- Quininehydrochloride,dihydrat
- S2502
- SC-05720
- ZX-AFC000703
References_____________________________________________________________________
(1) Bichali S, Brault D, Masserot C, Boscher C, Couec ML, Deslandes G, Pissard S, Leverger G, Vauzelle C, Elefant E, Rozé JC, Cortey A, Chenouard A. Maternal consumption of quinine-containing sodas may induce G6PD crises in breastfed children. Eur J Pediatr. 2017 Oct;176(10):1415-1418. doi: 10.1007/s00431-017-2998-5.
(2) Liles NW, Page EE, Liles AL, Vesely SK, Raskob GE, George JN. Diversity and severity of adverse reactions to quinine: A systematic review. Am J Hematol. 2016 May;91(5):461-6. doi: 10.1002/ajh.24314.
(3) «Report of the Scientific Committee for Food on Quinine.» (19 February 1988). In Food — Science and techniques. Reports of the Scientific Committee for Food (Twenty-first series).
(4) «Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from Commission on Flavouring Group Evaluation 35, (FGE.35) Three quinine salts from the Priority list from chemical group 30.»EFSA Journal (2008) 739, pagg. 1-18.
.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2019-06-03 08:51:01 | Chemical Risk: |