Il Lattato ferroso è un composto chimico, sale di ferro dell'acido lattico prodotto con sintesi chimica dall'acido lattico in due fasi: nella prima fase la reazione produce il lattato di bario e nella seconda lo ione di bario è sostituito con lo ione ferroso. Dal punto di vista ecologico è una sostanza non tossica, sicura per l'ambiente e che promuove la crescita delle alghe. Commercialmente si trova in forma diidrato e triidrato.
Il nome definisce la struttura della molecola
- Ferrous (Ferroso) - Indica la presenza di ferro in stato di ossidazione +2.
- Lactate (Lattato) - Deriva dall'acido lattico, un acido carbossilico.
Descrizione delle materie prime utilizzate nella produzione
- Acido lattico - Un acido carbossilico, spesso derivato dalla fermentazione di carboidrati.
- Solfato ferroso - Una fonte comune di ioni ferro (II).
Processo di sintesi
- Reazione - L'acido lattico reagisce con il solfato ferroso in una soluzione acquosa.
- Precipitazione - Il lattato ferroso precipita dalla soluzione e può essere raccolto tramite filtrazione.
- Purificazione ed essiccazione - Il precipitato viene lavato e successivamente essiccato per ottenere il lattato ferroso puro.
Si presenta in forma di polvere giallastra solubile in acqua.

A cosa serve e dove si usa
Alimentazione
E' inserito nella lista degli additivi alimentari con il numero E585 e con funzione di regolatore di acidità, antibatterico (1) e fortificatore ferroso facilmente assorbibile.

Viene utilizzato come fissativo per le olive mature, è utilizzato negli alimenti per lattanti (2), come integratore per carenza di ferro. Il lattato ferroso è GRAS per usi vari e generici negli Stati Uniti, senza alcuna limitazione se non le buone pratiche di fabbricazione. Il lattato ferroso può essere utilizzato come integratore alimentare e nutriente.
Medicina
Il Lattato ferroso è efficace nel correggere l'anemia come integratore e fornisce lo standard di 10 mg di ferro (3) e dall'industria farmaceutica come fonte di ferro terapeutico.
Cosmetica
Agente condizionante della pelle. Rappresenta il perno del trattamento topico della pelle in quanto ha la funzione di ripristinare, aumentare o migliorare la tolleranza cutanea a fattori esterni, compresa la tolleranza dei melanociti. La funzione più importante dell'agente condizionante è prevenire la disidratazione della pelle, ma il tema è piuttosto complesso e coinvolge emollienti ed umettanti che possono essere aggiunti nella formulazione.
Su questo ingrediente sono stati selezionati gli studi più rilevanti con una sintesi dei contenuti:
Lattato di ferro studi

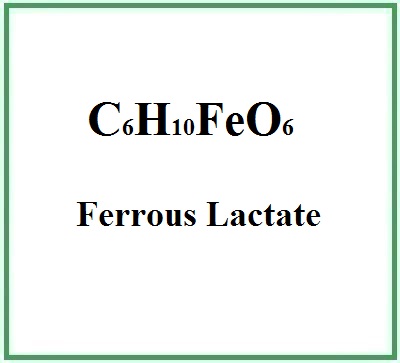
- Formula molecolare: C6H10FeO6
- Linear Formula [CH3CH(OH)COO]2Fe · xH2O
- Peso molecolare: 233.985 g/mol
- UNII: 5JU4C2L5A0
- CAS: 5905-52-2 85993-25-5
- EC Number: 227-608-0 289-080-8
- FEMA Number: 4699
- Beilstein Registry Number 6099658
Sinonimi:
- Ferrous Lactate
- Iron(II) lactate
- Iron(2+) lactate, (2:1)
- Iron(2+) lactate
- Lactic acid, iron(2+) salt (2:1)
- Propanoic acid, 2-hydroxy-, iron(2+) salt (2:1)
- Iron, bis((2S)-2-(hydroxy-kappaO)propanoato-kappaO)-, (T-4)-
- 2-hydroxypropanoate; iron(2+)
- (S)-Bis(lactato-O1,O2)iron
- Iron(2+) 2-hydroxypropanoate, (2:1)
Bibliografia_________________________________________________________________________
(1) Liang, Y., Zhang, L., Qu, Y., Li, H. and Shi, B., 2020. Antibacterial activity of buckwheat honey added with ferrous lactate against Pseudomonas aeruginosa. LWT, 117, p.108624.
(2) Federal Register / Vol. 81, No. 22 / Wednesday, February 3, 2016 / Rules and Regulations 5595
(3) Mosha, T.C., Laswai, H.H., Assey, J. and Bennink, M.R., 2014. Efficacy of a low-dose ferric-EDTA in reducing iron deficiency anaemia among underfive children living in malaria-holoendemic district of Mvomero, Tanzania. Tanzania Journal of Health Research, 16(2).
![]() Lattato di ferro
Lattato di ferro