Check the ingredients!
... live healthy!


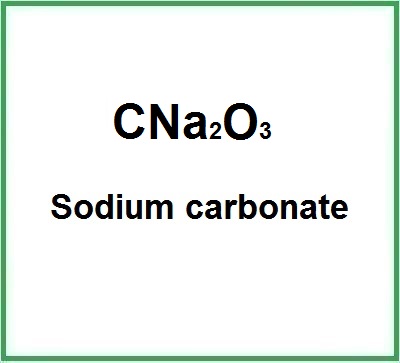
![]() Sodium carbonate
Sodium carbonate
Rating : 6
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Anti-inflammatory (1)Cons:
Avoid excessive amounts (1)10 pts from lu22
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Sodium carbonate studies" about Sodium carbonate Review Consensus 8 by lu22 (2319 pt) | 2021-Nov-29 18:02 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
The Effect of Concentration on the Cross-Linking and Gelling of Sodium Carbonate-Soluble Apple Pectins.
Gawkowska D, Cieśla J, Zdunek A, Cybulska J.
Molecules. 2019 Apr 25;24(8). pii: E1635. doi: 10.3390/molecules24081635.
A Novel Complex of Chitosan⁻Sodium Carbonate and Its Properties.
Qian J, Wang X, Shu J, Su C, Gong J, Xu Z, Jin J, Shi J.
Mar Drugs. 2018 Oct 30;16(11). pii: E416. doi: 10.3390/md16110416.
Side effects of powdered sodium carbonate (washing or 'Lectric' soda) used as an oral emetic agent in five dogs.
Watson AK, Indrawirawan YH.
Aust Vet J. 2019 May;97(5):157-161. doi: 10.1111/avj.12798.
New process for conversion of hazardous industrial effluent of ceramic industry into nanostructured sodium carbonate and their application in textile industry.
Dabas N, Yadav KK, Ganguli AK, Jha M.
J Environ Manage. 2019 Jun 15;240:352-358. doi: 10.1016/j.jenvman.2019.03.066
Thermodynamic Study of the Corrosion of Refractories by Sodium Carbonate.
Zhao Y, Cheng G, Xiang Y, Long F, Dong C.
Materials (Basel). 2018 Nov 6;11(11). pii: E2197. doi: 10.3390/ma11112197.
The role of sodium carbonate in PAM coagulation-flocculation for oil acidized wastewater treatment.
Qin J, Wang H, Qin C, Meng H, Qu W, Qian H.
Water Sci Technol. 2018 Jun;77(11-12):2677-2686. doi: 10.2166/wst.2018.224
Effects of sodium carbonate and potassium carbonate on colloidal properties and molecular characteristics of konjac glucomannan hydrogels.
Liu X, Gan J, Nirasawa S, Tatsumi E, Yin L, Cheng Y.
Int J Biol Macromol. 2018 Oct 1;117:863-869. doi: 10.1016/j.ijbiomac.2018.05.176
Structure and Liquid Fragility in Sodium Carbonate.
Wilson M, Ribeiro MCC, Wilding MC, Benmore C, Weber JKR, Alderman O, Tamalonis A, Parise JB.
J Phys Chem A. 2018 Feb 1;122(4):1071-1076. doi: 10.1021/acs.jpca.7b10712.
Synergy of lignocelluloses pretreatment by sodium carbonate and bacterium to enhance enzymatic hydrolysis of rice straw.
Shen Z, Zhang K, Si M, Liu M, Zhuo S, Liu D, Ren L, Yan X, Shi Y.
Bioresour Technol. 2018 Feb;249:154-160. doi: 10.1016/j.biortech.2017.10.008.
Fabrication of calcite blocks from gypsum blocks by compositional transformation based on dissolution-precipitation reactions in sodium carbonate solution.
Ishikawa K, Kawachi G, Tsuru K, Yoshimoto A.
Mater Sci Eng C Mater Biol Appl. 2017 Mar 1;72:389-393. doi: 10.1016/j.msec.2016.11.093
Impact of a sodium carbonate spray combined with professional oral hygiene procedures in patients with Sjögren's syndrome: an explorative study.
Gambino A, Broccoletti R, Cafaro A, Cabras M, Carcieri P, Arduino PG.
Gerodontology. 2017 Jun;34(2):208-214. doi: 10.1111/ger.12250.
Comparative toxicity of sodium carbonate peroxyhydrate to freshwater organisms.
Geer TD, Kinley CM, Iwinski KJ, Calomeni AJ, Rodgers JH Jr.
Ecotoxicol Environ Saf. 2016 Oct;132:202-11. doi: 10.1016/j.ecoenv.2016.05.037.
Reducing biomass recalcitrance via mild sodium carbonate pretreatment.
Mirmohamadsadeghi S, Chen Z, Wan C.
Bioresour Technol. 2016 Jun;209:386-90. doi: 10.1016/j.biortech.2016.02.096.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Sodium carbonate Review Consensus 10 by lu22 (2319 pt) | 2024-Oct-07 17:30 |
| Read the full Tiiip | (Send your comment) |
Sodium carbonate is a chemical compound, the sodium salt of carbonic acid and it is commonly known as soda ash or washing soda. It is used in various industries, including the food industry, where it acts as a pH regulator and leavening agent. In everyday life, it can be used as a food additive (E500) and also in cleaning products, such as laundry detergents.
Sodium Carbonate, also known as soda ash or washing soda, is an inorganic chemical compound with the formula Na2CO3. It is used in various industrial, cosmetic, and household applications, including detergents, personal care products, and as a pH regulator. Sodium Carbonate is particularly valued for its ability to alkalinize and balance the pH in cosmetic formulations and for its detergent and whitening properties.
Chemical Composition and Structure
Sodium Carbonate consists of sodium ions (Na+) and carbonate ions (CO3²-). It is a salt derived from carbonic acid and appears naturally as a white crystalline solid, which easily dissolves in water. Its chemical structure makes it highly alkaline, making it effective in neutralizing acids and regulating pH in various formulations.
Physical Properties
It typically appears as a white or crystalline powder that is odorless. It is water-soluble and, when dissolved, produces a strongly alkaline solution. These properties make it particularly useful in cleaning products and as a pH regulator in various cosmetic and personal care formulations.
Production Process
Sodium Carbonate can be produced synthetically through the Solvay process, which involves the reaction of sodium chloride (common salt) and calcium carbonate (limestone). However, it can also be extracted naturally from trona deposits, a mineral that contains hydrated sodium carbonate. After extraction, it is purified for use in cosmetic and industrial applications.
The name describes the structure of the molecule.
Description of raw materials used in production.
Step-by-step summary of industrial production process.
Form and color.
Sodium carbonates typically appear as a white crystalline solid, which can be either pulverized or granulated. They are water soluble and alkaline in nature, with a molecular structure that allows them to neutralize acids and perform a wide range of functions in various industrial and domestic applications. In particular, sodium bicarbonate (a form of sodium carbonate) is also known as baking soda, and is widely used not only in the food industry but also as a common household cleaning agent due to its neutralizing and cleaning properties.

Food
Ingredient included in the list of European food additives as E500 with a leavening function.
In the list of European food additives is specified as follows:
Sodium carbonate has multiple uses in the industrial sector of building materials, food industry, chemical industry with daily chemicals, metallurgy, textiles, pharmaceuticals, medicine and other sectors.
In the manufacturing process of detergents and soaps it is used as a filler and to make the product smoother.
It is adjuvant in the production of chemical products such as sodium silicate, sodium bicarbonate and percarbonate, chromate and sodium dichromate.
The glass industry needs 0.2 tons of sodium carbonate per ton of glass because, in the glass manufacturing process it reduces the melting temperature of the sand used in glass formation and aids in the shaping and workability of glassware such as tableware, optical glass and float glass.
Sodium carbonate can reduce the corrosive effect of alkali dust on refractory materials and extend furnace life.
In the food industry it acts as a leavening, buffering and dough softening agent in baked goods and especially in cakes and pastries as well as in soy sauce, bread, amino acid production. When mixed with alkaline water and added to dough has the property of increasing elasticity and ductility. Sodium carbonate can also be used to produce MSG, Monosodium Glutamate.
In the pharmaceutical industry it is used as osmotic laxative and antacid. It is inserted into medicinal tablets as a filler, a substance used to increase the volume of the mass to be compressed to the desired volume.
In the metalworking industry, sodium carbonate is used for electrolytic coppering, electrolytic polishing of aluminum and alloys, chemical and electrochemical degreasing, chemical oxidation of aluminum, corrosion of aluminum, rust prevention between processes, electrolytic degradation, sealing after phosphating. It also serves for chromium plating and subsequent removal of chromium oxide film, copper pre-plating, steel plating and in the electrolyte plating of steel alloy.
In metallurgical industry, it is desulfurization agent in steel production and antimony smelting.
In the tanning industry it neutralizes chrome tanned leather, degreases leather, improves the alkalinity of chrome tanning bath.
Also used as a test to calibrate the acid in quantitative analysis, co-solvent analysis of silica in cement, glucose analysis in urine and blood, test for the determination of copper, lead, zinc, aluminum, sulfur. Metallographic analysis etc.
For the production of lactulose, it has proved to be effective by considerably reducing the production time (1).
It is used in the preparation and cooking of patties and hamburgers to improve their elasticity, hardness and cohesion (2).
It has been used as an efficient and inexpensive alkaline catalyst for pretreatment of corn straw and lignocellulosic biomass (3).
It is used to improve the tolerance of fruit (in this case pears) to frost damage (4).
It is an anti-inflammatory and is also used as a tooth-cleaning agent (5).
Food safety
A 2024 study warns about the risk of developing cancer with high intakes of emulsifiers, (including E440, Pectin, E471 mono- and diglycerides of fatty acids, carrageenan, E407, sodium carbonate E500) (6).
Health and Safety Considerations
Safety in Use
Sodium Carbonate is generally considered safe for use in cosmetic and personal care products. However, due to its high alkalinity, it can cause irritation if used in high concentrations or on sensitive skin. It should be used with caution in formulations intended for direct skin contact.
Allergic Reactions
Allergic reactions to Sodium Carbonate are rare. However, in individuals with sensitive skin or when exposed to high concentrations, it may cause skin irritation. It is always recommended to follow usage instructions and, in the case of sensitive skin, perform a patch test before use.
Toxicity and Carcinogenicity
There is no evidence that Sodium Carbonate is toxic or carcinogenic. It is considered safe for use in cosmetic and household products when used at appropriate concentrations. However, ingestion of large amounts can cause gastrointestinal irritation.
Environmental and Safety Considerations
Sodium Carbonate is a natural and biodegradable compound, meaning it has minimal environmental impact. It is environmentally safe if used and disposed of properly. However, excessive use in aquatic environments may alter water pH, potentially harming aquatic life.
Regulatory Status
Sodium Carbonate is approved for use in cosmetics and personal care products in many regions, including the European Union and the United States. It is considered safe at the concentrations commonly used in these products.
The most relevant studies on this ingredient have been selected with a summary of their contents:
Synonyms:
References______________________________________________________________________
(1) Seo YH, Park GW, Han JI. Efficient lactulose production from cheese whey using sodium carbonate. Food Chem. 2015 Apr 15;173:1167-71. doi: 10.1016/j.foodchem.2014.10.109.
(2) Parlak O, Zorba O, Kurt S. Modelling with response surface methodology of the effects of egg yolk, egg white and sodium carbonate on some textural properties of beef patties. J Food Sci Technol. 2014 Apr;51(4):780-4. doi: 10.1007/s13197-011-0552-4.
(3) Kim I, Rehman MS, Han JI. Enhanced glucose yield and structural characterization of corn stover by sodium carbonate pretreatment. Bioresour Technol. 2014;152:316-20. doi: 10.1016/j.biortech.2013.10.069.
(4) D'Aquino S, Barberis A, Continella A, La Malfa S, Gentile A, Schirra M. Individual and combined effects of postharvest dip treatments with water at 50 degrees C, soy lecithin and sodium carbonate on cold stored cactus pear fruits. Commun Agric Appl Biol Sci. 2012;77(3):207-17.
(5) Turker SB, Sener ID, Koçak A, Yilmaz S, Ozkan YK. Factors triggering the oral mucosal lesions by complete dentures. Arch Gerontol Geriatr. 2010 Jul-Aug;51(1):100-4. doi: 10.1016/j.archger.2009.09.001.
(6) Sellem, L., Srour, B., Javaux, G., Chazelas, E., Chassaing, B., Viennois, E., ... & Touvier, M. (2024). Food additive emulsifiers and cancer risk: Results from the French prospective NutriNet-Santé cohort. Plos Medicine, 21(2), e1004338.
Abstract. Emulsifiers are widely used food additives in industrially processed foods to improve texture and enhance shelf-life. Experimental research suggests deleterious effects of emulsifiers on the intestinal microbiota and the metabolome, leading to chronic inflammation and increasing susceptibility to carcinogenesis. However, human epidemiological evidence investigating their association with cancer is nonexistent. This study aimed to assess associations between food additive emulsifiers and cancer risk in a large population-based prospective cohort.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-02-09 12:37:11 | Chemical Risk: |