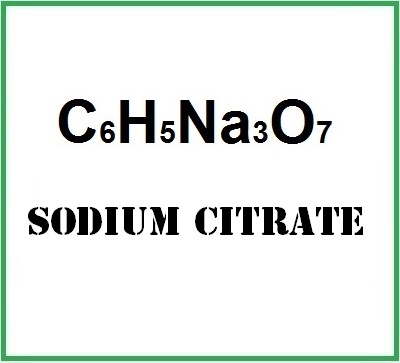
![]() Sodium citrate
Sodium citrate
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antioxidant (1)10 pts from Flight444
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Sodium citrate studies" about Sodium citrate Review Consensus 8 by Flight444 (3416 pt) | 2019-May-24 22:10 |
| Read the full Tiiip | (Send your comment) |
Influence of Sodium Citrate Supplementation after Dehydrating Exercise on Responses of Stress Hormones to Subsequent Endurance Cycling Time-Trial in the Heat.
Suvi S, Mooses M, Timpmann S, Medijainen L, Unt E, Ööpik V.
Medicina (Kaunas). 2019 Apr 12;55(4). pii: E103. doi: 10.3390/medicina55040103
Sodium citrate contributes to the platelet storage lesion.
Getz TM, Turgeon A, Wagner SJ.
Transfusion. 2019 Feb 22. doi: 10.1111/trf.15213
Does Sodium Citrate Cause the Same Ergogenic Effect As Sodium Bicarbonate on Swimming Performance?
Kumstát M, Hlinský T, Struhár I, Thomas A.
J Hum Kinet. 2018 Dec 31;65:89-98. doi: 10.2478/hukin-2018-0022
Sodium Citrate Increases Expression and Flux of Mg2+ Transport Carriers Mediated by Activation of MEK/ERK/c-Fos Pathway in Renal Tubular Epithelial Cells.
Takashina Y, Manabe A, Hasegawa H, Matsunaga T, Endo S, Ikari A.
Nutrients. 2018 Sep 20;10(10). pii: E1345. doi: 10.3390/nu10101345.
A case of severe metformin-associated lactic acidosis treated with CVVHDF and regional anticoagulation with sodium citrate.
Ferrario M, Apicella A, Della Morte M, Beretta E.
G Ital Nefrol. 2018 Sep;35(5). pii: 2018-vol5.
A randomized controlled clinical trial of 4% sodium citrate versus heparin as locking solution for temporary dialysis catheters among hemodialysis patients
.
Abdel Azim ABE, ElSaid TW, El Said HW, Hemida W, Zaghlool S, Ramadan A, Gouda Z, El Masry S, Reda W, Ali HM.
Clin Nephrol. 2018 Nov;90(5):341-349. doi: 10.5414/CN109162.
Validation of a New Small-Volume Sodium Citrate Collection Tube for Coagulation Testing in Critically Ill Patients with Coagulopathy.
Adam EH, Zacharowski K, Hintereder G, Zierfuß F, Raimann F, Meybohm P.
Clin Lab. 2018 Jun 1;64(6):1083-1089. doi: 10.7754/Clin.Lab.2018.171008.
Effects of sodium citrate, citric acid and lactic acid on human blood coagulation.
Scaravilli V, Di Girolamo L, Scotti E, Busana M, Biancolilli O, Leonardi P, Carlin A, Lonati C, Panigada M, Pesenti A, Zanella A.
Perfusion. 2018 Oct;33(7):577-583. doi: 10.1177/0267659118777441. Epub 2018 May 22.
Sodium citrate inhibits the proliferation of human gastric adenocarcinoma epithelia cells.
Xia Y, Zhang X, Bo A, Sun J, Li M.
Oncol Lett. 2018 May;15(5):6622-6628. doi: 10.3892/ol.2018.8111. Epub 2018 Feb 23.
Impact of sodium citrate ingestion during recovery after dehydrating exercise on rehydration and subsequent 40-km cycling time-trial performance in the heat.
Suvi S, Mooses M, Timpmann S, Medijainen L, Narõškina D, Unt E, Ööpik V.
Appl Physiol Nutr Metab. 2018 Jun;43(6):571-579. doi: 10.1139/apnm-2017-0584. Epub 2018 Jan 11
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Sodium citrate Review Consensus 10 by Flight444 (3416 pt) | 2025-Feb-04 17:19 |
| Read the full Tiiip | (Send your comment) |
Sodium citrate or Citrosodine, is a chemical compound, inorganic salt of citric acid (α and β-hydroxytricarboxylic acid).
The name describes the structure of the molecule:
- Sodium indicates the presence of sodium ions in the molecule.
- Citrate is derived from the word "citric." and indicates that the molecule is a salt or ester of citric acid, a tricarboxylic acid found in many citrus fruits.
Description of the raw materials used in its production:
- Citric Acid - Citric acid is an organic acid found in many natural sources such as citrus fruits. It can be obtained from these sources or produced through fermentation or chemical synthesis.
- Sodium - Sodium is a chemical element present in many compounds. In the case of sodium citrate, citric acid reacts with sodium hydroxide (NaOH) or sodium carbonate (Na2CO3) to form sodium citrate. This chemical reaction involves the neutralization of citric acid with sodium.
Industrial chemical synthesis step-by-step:
- Citric Acid Preparation - The industrial production of citric acid takes place by mycological fermentation of raw sugar from Aspergillus niger and subsequent crystallisation with alkaline solutions.
- Reaction with Sodium Hydroxide - Citric acid reacts with sodium hydroxide or sodium carbonate in a controlled reaction to form sodium citrate. This reaction involves the neutralization of the acidity of citric acid with sodium, resulting in the formation of a sodium salt.
- Purification - The crude sodium citrate solution obtained from the reaction is purified to remove impurities and unwanted by-products. This can be done through processes such as filtration, crystallization, or other purification methods.
- Drying and Packaging - The purified sodium citrate is dried to remove residual moisture and then packaged for distribution and use in various applications.
It appears as small crystals or a fine, white powder, soluble in water.

What it is for
Citrate plays a notable role in cellular metabolism and is an important intermediate in the tricarboxylic acid cycle. In medicine it is used as an anticoagulant in stored blood, and for alkalinization of urine in the prevention of kidney stones.
Cosmetics
- pH adjuster. This ingredient tends to restore the pH of a cosmetic formulation to its optimal value. The correct pH value is an essential determinant for lipid synthesis in the stratum corneum. The average physiological pH value of the face ranges between 5.67 and 5.76. The hair fibre has a pH value of 3.67.
- Buffering agent. Iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
- Chelating agent. It has the function of preventing unstable reactions and improving the bioavailability of chemical components within a product, and removes calcium and magnesium cations that can cause cloudiness in clear liquids.
- Fragrance. It plays a very important role in the formulation of cosmetic products as it allows perfume to be enhanced, masked or added to the final product, improving its commercial viability. The consumer always expects to find a pleasant scent in a cosmetic product.
Food
Labeled as E331 on the food additives list as an acidity regulator to restore pH to normal, it has also been shown to improve dough in baked goods as adding sodium citrate to dough produced improved gluten aggregation and increased gluten yield over the control by raising the pH and approaching the isoelectric point of glutenin and gliadin. In addition, sodium citrate resulted in an increased particle size distribution of the glutenin macropolymer (1).
For example, in cheese, the inclusion of sodium citrate resulted in expansion of the protein matrix and increase in cheese hardness.
It is used in beverages as an acidity corrector and antioxidant.
Medical
In medicine, infused sodium citrate resulted in acidification and anticoagulation of blood similar to lactic acid and sodium citrate, respectively, without affecting blood gas analysis (2).
Kidney stones affect people all over the world and have a high recurrence rate even with treatment. Citrate salts prevent new stone formation and reduce further stone growth in patients with residual stones that contain predominantly oxalate (3).
The results of this study demonstrated that treatment with sodium citrate at higher concentrations or for longer periods exerts a cytotoxic effect on AGS cells through induction of the intrinsic apoptosis pathway and alteration of the levels of certain cytokines (4).
In toothpastes, the addition of sodium citrate improved dentin sensitivity (5).
In cosmetics, the inclusion of sodium citrate in the formula allows to maintain the pH at acceptable levels and to regulate the overall acidity level.
For more information:
 |  |
 |  |
Optimal typical characteristics of the commercial product sodium citrate
| Appearance | Small crystals or a fine, white powder. |
| Melting Point | 300°C |
| Purity | 99% |
| Density | 1.008 g/mL at 20 °C |
| Storage temperature | 2-8°C |
| Shelf life | 24 months |
Where to buy sodium citrate
- Molecular Formula : C6H5Na3O7 C6H5O7. 3Na Na3C6H5O7
- Linear Formula : HOC(COONa)(CH2COONa)2 · 2H2O
- Molecular Weight : 258.068 g/mol
- CAS : 68-04-2
- EC Number : 200-675-3 213-618-2 200-675-3
- UNII RS7A450LGA
- DSSTox Substance ID: DTXSID2026363 DTXSID1027348
- MDL number MFCD00012462
- PubChem Substance ID 24899674
- InChl 1S/C6H8O7.3Na/c7-3(8)1-6(13,5(11)12)2-4(9)10;;;/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12);;;/q;3*+1/p-3
- InChI Key HRXKRNGNAMMEHJ-UHFFFAOYSA-K
- SMILES C(C(=O)[O-])C(CC(=O)[O-])(C(=O)[O-])O.[Na+].[Na+].[Na+]
- IUPAC trisodium;2-hydroxypropane-1,2,3-tricarboxylate
- ChEBI 53258
- FEMA 3026
- NACRES NA.25
Synonyms :
- Trisodium citrate
- Trisodium citrate, anhydrous
- Citrosodine
- Sodium citrate anhydrous
- Trisodium 2-hydroxy-1,2,3-propanetricarboxylate
- 1,2,3-Propanetricarboxylic acid, 2-hydroxy-, trisodium salt
References_____________________________________________________________________
(1) Amiri A, Farshi-Marandi P, Shahedi M. Impact of sodium citrate on structural properties of gluten. J Food Sci Technol. 2019 Feb;56(2):1090-1093. doi: 10.1007/s13197-019-03571-6.
(2) Scaravilli V, Di Girolamo L, Scotti E, Busana M, Biancolilli O, Leonardi P, Carlin A, Lonati C, Panigada M, Pesenti A, Zanella A. Effects of sodium citrate, citric acid and lactic acid on human blood coagulation. Perfusion. 2018 Oct;33(7):577-583. doi: 10.1177/0267659118777441.
(3) Phillips R, Hanchanale VS, Myatt A, Somani B, Nabi G, Biyani CS. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst Rev. 2015 Oct 6;(10):CD010057. doi: 10.1002/14651858.CD010057.pub2.
(4) Xia Y, Zhang X, Bo A, Sun J, Li M. Sodium citrate inhibits the proliferation of human gastric adenocarcinoma epithelia cells. Oncol Lett. 2018 May;15(5):6622-6628. doi: 10.3892/ol.2018.8111.
(5) Clark DC, Al-Joburi W, Chan EC. The efficacy of a new dentifrice in treating dentin sensitivity: effects of sodium citrate and sodium fluoride as active ingredients. J Periodontal Res. 1987 Mar;22(2):89-93. doi: 10.1111/j.1600-0765.1987.tb01545.x.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2024-04-09 19:18:06 | Chemical Risk: |