Potassium lactate is the potassium salt of lactic acid.
The name describes the structure of the molecule:
- Potassium refers to the potassium ion (K+), which is an alkaline metal.
- Lactate refers to the lactate ion (C3H5O3-), which is obtained from lactic acid (C3H6O3). Lactic acid is an organic acid that plays a key role in different biochemical processes.
The synthesis process takes place in different steps:
- Preparation. The first stage of potassium lactate synthesis is the preparation of lactic acid through the fermentation of sugars by some bacteria, such as Lactobacillus, or through chemical synthesis.
- Neutralization reaction. Lactic acid is neutralized with potassium hydroxide with a reaction that produces potassium lactate and water. The reaction is typically carried out in an aqueous solution.
- Crystallization. The resulting solution is cooled, producing the crystallization of potassium lactate from the solution. The crystals are then collected through filtration.
- Purification. The collected crystals are purified, through recrystallization by dissolving the crystals in a minimum amount of hot water and then the slow cooling of the solution to form purer crystals of potassium lactate.
- Drying. Purified crystals are dried to remove residual water.
- Quality control. The final product is then tested to ensure it meets the required specifications. This may involve testing for parameters such as purity, pH and moisture content.
Industrially it appears as a clear liquid.

What it is used for and where
Food
It is an ingredient labelled with the number E326 in the list of European food additives as a preservative.
Cosmetics
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Humectant. Hygroscopic compound used to minimise water loss in the skin and to prevent it from drying out by facilitating faster and greater absorption of water into the stratum corneum of the epidermis. The epidermis is the most superficial of the three layers that make up human skin (epidermis, dermis and hypodermis) and is the layer that maintains hydration in all three layers. In turn, the epidermis is composed of five layers: horny, the most superficial, granular, spinous, shiny, and basal. Humectants have the ability to retain the water they attract from the air in the stratum corneum and have the function of moisturising the skin. They are best used before emollients, which are oil-based.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
The most relevant studies on this ingredient have been selected with a summary of their contents:
Potassium lactate studies
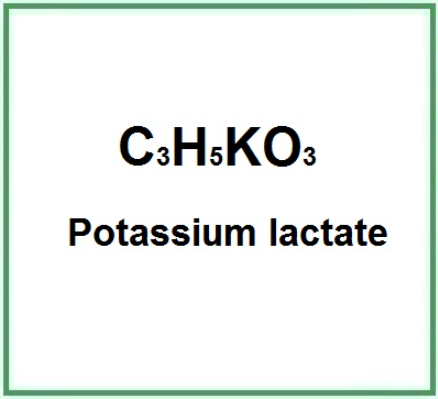
- Molecular Formula: C3H5O3K C3H5KO3
- Molecular Weight: 128.168 g/mol
- CAS: 996-31-6 85895-78-9
- EC Number: 213-631-3 288-752-8
Synonyms:
- Lactic acid, potassium salt
- Monopotassium lactate
- Monopotassium 2-hydroxypropanoate acid
- Potassium alpha-hydroxypropionate
- Monopotassium 2-hydroxypropanoate
- potassium 2-hydroxypropanoate
![]() Potassium lactate
Potassium lactate