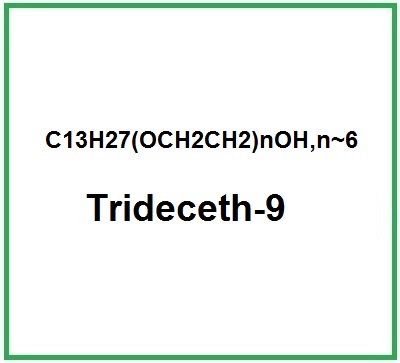
![]() Trideceth-9
Trideceth-9
Rating : 5.7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Ethoxylated chemical compound (1)17 pts from A_Partyns
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "PEG-9 Tridecyl ether Trideceth-9 st" about Trideceth-9 Review Consensus 7 by Al222 (20707 pt) | 2021-Nov-20 18:46 |
| Read the full Tiiip | (Send your comment) |
PEG-9 Tridecyl ether Trideceth-9 as well as in cosmetic products, it is used for the removal of heavy metals such as Cadmium from wastewater (1).
It is considered safe by the CIR expert committee (2).
References_________________________
(1) Removal of trace Cd2+ from aqueous solution by foam fractionation.
Lu J, Li Y, Zhang S, Sun Y.
J Hazard Mater. 2015 Apr 9;286:466-73. doi: 10.1016/j.jhazmat.2015
(2) CIR EXPERT PANEL MEETING
DECEMBER 13-14, 2010
(3) Use of polyethylene glycol in functional constipation and fecal impaction.
Mínguez M, López Higueras A, Júdez J.
Rev Esp Enferm Dig. 2016 Nov
Effectiveness of senna vs polyethylene glycol as laxative therapy in children with constipation related to anorectal malformation.
Santos-Jasso KA, Arredondo-García JL, Maza-Vallejos J, Lezama-Del Valle P.
J Pediatr Surg. 2016 Oct
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Trideceth-9 Review Consensus 17 by A_Partyns (12948 pt) | 2023-Sep-10 16:08 |
| Read the full Tiiip | (Send your comment) |
Trideceth-9 is a chemical compound, 13 carbon chains alkyl polyethylene glycol (PEG (Polyethylene glycol)) ether of ethoxylated fatty Tridecyl alcohol (9 mol).
The name describes the structure of the molecule:
- Tridec refers to tridecyl alcohol, a fatty alcohol with a chain of 13 carbons, which forms the basis of the molecule.
- -eth indicates that ethylene oxide units have been added to the molecule.
- -9 means that, on average, 9 ethylene oxide units are added to each molecule of tridecyl alcohol.
In terms of raw materials, Trideceth-9 is primarily produced from two components:
- Tridecanol - This is a fatty alcohol with a chain of 13 carbon atoms. It is primarily derived from natural sources such as vegetable oils.
- Ethylene oxide - This is a chemical compound used to ethoxylate the fatty alcohol. The ethylene oxide reacts with the alcohol to form a polyethylene glycol ether, which is Trideceth-9.
The synthesis process takes place in different steps:
- Preparation of tridecyl alcohol. Tridecyl alcohol, a fatty alcohol with a chain of 13 carbon atoms, is derived from natural sources or synthesised from petrochemical precursors.
- Ethoxylation. Tridecyl alcohol is reacted with ethylene oxide in a process called ethoxylation. This involves the addition of ethylene oxide units to the alcohol, forming an ether. The number of ethylene oxide units added can be controlled to obtain different products; in the case of Trideceth-9, an average of 9 ethylene oxide units are added.
- Neutralization. The product can be neutralized if necessary.
- Purification. The product may be purified via distillation or other methods to remove any impurities.
Safety
The term 'eth' refers to the ethoxylation reaction with ethylene oxide after which residues of ethylene oxide and 1,4-dioxane, chemical compounds considered carcinogenic, may remain. The degree of safety therefore depends on the degree of purity of the compound obtained. At present, no manufacturer is known to provide this information on the label.
It appears as a colourless liquid

What it is used for and where
Cosmetics
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Emulsion stabilizer. Emulsions are thermodynamically unstable. Emulsion stabilisers improve the formation and stability of single and double emulsions. It should be noted that in the structure-function relationship, molar mass plays an important role.
Other uses
Trideceth-9 as well as in cosmetic products, is used for the removal of heavy metals such as Cadmium from wastewater (1).
Safety
It is considered safe by the Committee of Experts CIR (2).
- Molecular Formula C15H32O2
- Molecular Weight 244.419 g/mol
- CAS 24938-91-8 38471-49-7
- EC number 607-463-3 500-241-6
Synonyms:
- Trideceth-5; Trideceth-6; Trideceth-7; Trideceth-8; Trideceth-9; Trideceth-10; Trideceth-11; Trideceth-12; Trideceth-15; Trideceth-20; Trideceth-50; Ethoxylated tridecyl alcohol; Polyoxyethylene tridecyl ether; Tridecyl alcohol, ethoxylated
References________________________________________________________________________
(1) Lu J, Li Y, Zhang S, Sun Y. Removal of trace Cd2+ from aqueous solution by foam fractionation. J Hazard Mater. 2015 Apr 9;286:466-73. doi: 10.1016/j.jhazmat.2015.01.029.
(2) CIR EXPERT PANEL MEETING DECEMBER 13-14, 2010
_____________________________________________________________________________
And a premise on PEG.
Since the PEG (1) family is numerous and is found in many cosmetic, cleaning and medicinal products and others, we need a cognitive premise on the subject that is rather complex from the point of view of safety because these products not only come into contact with the skin but, as in the case of medicine, they are also ingested.
PEG or polyethylene glycols polymerise the condensed ethylene oxide and water and are called polyethylene glycols, but in reality, they are complex chemical components, polymers bound together. For example, plastic is polyethylene and has a hard consistency, while polyethylene aggregated to the glycol forms a liquid.
The number that appears after the initials PEG represents the molecular weight and the higher this number is, the less it penetrates the skin.
Here below are some studies in Medicine that refer to the use of PEG Polyethylene glycol in various fields.
Intestine
Polyethylene glycol with or without electrolytes is effective for the treatment of functional constipation, both in adults and in paediatric patients, with great safety and tolerability. These preparations are the most effective osmotic laxatives (more than lactulose) and are the first-line treatment for functional constipation in the short- and long-term. They are as effective as enemas in faecalomas, avoid the need for hospitalisation and are well tolerated by patients (especially when given without electrolytes) (2).
In the preparation for colonoscopy, polyethylene glycol tablets confirmed efficacy, acceptability, tolerance and safety similar to those of sodium phosphate (3).
For peripheral nerve repair (4).
Eyes
Dry eye syndrome is a disorder that affects 5-34% of the world's adult population with reduced quality of life. Artificial or lubricating tears are the most used therapy for treating this condition due to their low side effects profile, which attempt to modify the properties of the tear film. Polyethylene glycol has demonstrated clinical efficacy in the treatment of this condition (5).
Brain
Polyethylene glycol facilitates the neuroprotective effects of magnesium in head injuries (6).
Tumors
For transarterial chemoembolization, Polyethylene glycol is effective and safe for the treatment of liver cancer, as indicated by good tolerability, quality of life and high tumour response (7).
Cosmetics
Many types of PEG are hydrophilic and are used as creams, topical dermatological preparations and in cosmetic products such as surfactants, emulsifiers, detergents, humectants and skin conditioners.
Safety varies from type to type given the structural complexity (8).
References___________________________________________________________________
(1) Fruijtier-Pölloth C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology. 2005 Oct 15;214(1-2):1-38. doi: 10.1016/j.tox.2005.06.001.
(2) Mínguez M, López Higueras A, Júdez J. Use of polyethylene glycol in functional constipation and fecal impaction. Rev Esp Enferm Dig. 2016 Dec;108(12):790-806. doi: 10.17235/reed.2016.4571/2016.
Santos-Jasso KA, Arredondo-García JL, Maza-Vallejos J, Lezama-Del Valle P. Effectiveness of senna vs polyethylene glycol as laxative therapy in children with constipation related to anorectal malformation. J Pediatr Surg. 2017 Jan;52(1):84-88. doi: 10.1016/j.jpedsurg.2016.10.021.
(3) Chaussade S, Schmöcker C, Toulemonde P, Muñoz-Navas M, O'Mahony V, Henri F. Phosphate tablets or polyethylene glycol for preparation to colonoscopy? A multicentre non-inferiority randomized controlled trial. Surg Endosc. 2017 May;31(5):2166-2173. doi: 10.1007/s00464-016-5214-1.
Tsunoda T, Sogo T, Iwasawa K, Umetsu S, Oikawa-Kawamoto M, Inui A, Fujisawa T. Feasibility and safety of bowel cleansing using low-volume polyethylene glycol with ascorbic acid before pediatric colonoscopy: A pilot study. Dig Endosc. 2017 Mar;29(2):160-167. doi: 10.1111/den.12756.
(4) Hoffman AN, Bamba R, Pollins AC, Thayer WP. Analysis of polyethylene glycol (PEG) fusion in cultured neuroblastoma cells via flow cytometry: Techniques & optimization. J Clin Neurosci. 2017 Feb;36:125-128. doi: 10.1016/j.jocn.2016.10.032.
(5) Pérez-Balbuena AL, Ochoa-Tabares JC, Belalcazar-Rey S, Urzúa-Salinas C, Saucedo-Rodríguez LR, Velasco-Ramos R, Suárez-Sánchez RG, Rodríguez-Carrizalez AD, Oregón-Miranda AA. Efficacy of a fixed combination of 0.09 % xanthan gum/0.1 % chondroitin sulfate preservative free vs polyethylene glycol/propylene glycol in subjects with dry eye disease: a multicenter randomized controlled trial. BMC Ophthalmol. 2016 Sep 20;16(1):164. doi: 10.1186/s12886-016-0343-9.
Labetoulle M, Messmer EM, Pisella PJ, Ogundele A, Baudouin C. Safety and efficacy of a hydroxypropyl guar/polyethylene glycol/propylene glycol-based lubricant eye-drop in patients with dry eye. Br J Ophthalmol. 2017 Apr;101(4):487-492. doi: 10.1136/bjophthalmol-2016-308608.
(6) Busingye DS, Turner RJ, Vink R. Combined Magnesium/Polyethylene Glycol Facilitates the Neuroprotective Effects of Magnesium in Traumatic Brain Injury at a Reduced Magnesium Dose. CNS Neurosci Ther. 2016 Oct;22(10):854-9. doi: 10.1111/cns.12591.
(7) Aliberti C, Carandina R, Sarti D, Mulazzani L, Catalano V, Felicioli A, Coschiera P, Fiorentini G. Hepatic Arterial Infusion of Polyethylene Glycol Drug-eluting Beads for Primary and Metastatic Liver Cancer Therapy. Anticancer Res. 2016 Jul;36(7):3515-21.
(8) Jang HJ, Shin CY, Kim KB. Safety Evaluation of Polyethylene Glycol (PEG) Compounds for Cosmetic Use. Toxicol Res. 2015 Jun;31(2):105-36. doi: 10.5487/TR.2015.31.2.105.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-01-08 18:38:01 | Chemical Risk: |