E163 (Anthocyanins) is an approved food additive.
Anthocyanins belong to the phytochemical group of Flavonoids and are found in nature, in fruit, vegetables, tea, red wine they are recognizable by their blue pigmentation. Anthocyanins have strong antioxidant and antimicrobial activity, and their colour depends on the degree of hydroxylation/methylation, on the pH level (with flowers over time they tend to become darker due to transporters that carry potassium ions instead of protons) and from chelation with metals (e.g. the addition of a metal to hydrangeas makes it turn blue).
Anthocyanins belong to the flavonoid family. These molecules consist of a molecule of benzene fused with a pyran (a heterocyclic ring containing oxygen), in turn linked with a phenyl group, which can then be linked to different substituents. This complex molecule is called the flavy cation which is the basic structure of all anthocyanins.

Flavonoids, perhaps the single most important group of phenolic compounds in food, comprise a group of over 4000 aromatic vegetable compounds. In this group there are:
- anthocyanins (cyanidin, pelargonidine, petunidin)
- flavonols (quercetin, kaempferol)
- flavones (luteolin, apigenin)
- flavanones (myricetin, naringin, esperetin, naringenin)
- flavan-3-oils (catechin, epicatechin, gallocatechin)
- isoflavones (genistein, daidzein)
Chemical Composition and Structure
Anthocyanins are water-soluble compounds found in the form of glycosides, which are bound to sugars that stabilize the structure and increase solubility. Their chemical structure contains an aromatic ring that enables them to absorb light, producing bright colors. The color of anthocyanins varies depending on the pH: in an acidic environment, they tend to be red; in a neutral environment, they are more purple; and in an alkaline environment, they turn blue.
Physical Properties
Anthocyanins appear as a water-soluble powder that changes color based on the pH of the solution. They are stable at low temperatures but can degrade when exposed to heat and light. Their ability to produce a wide range of colors makes them ideal for use as natural food colorants in products such as beverages, candies, ice cream, and jams.
Production Process
Anthocyanins are extracted from natural sources, primarily from pigment-rich fruits and vegetables, through an aqueous or solvent extraction process. The product is then purified to remove impurities and concentrated for use in the food and cosmetic industries.
Applications
Food Industry: Anthocyanins (E163) are used to provide natural colors to products such as beverages, candies, yogurt, ice cream, and jams. Their natural origin makes them particularly valued in organic or natural formulations.
Cosmetics: They are used in lipsticks, eyeshadows, and other makeup products for their natural coloring properties and ability to provide vibrant shades.
Colorant. This ingredient has the primary function of colouring the solution in which it is inserted in a temporary, semi-permanent or permanent manner, either alone or in the presence of the complementary components added for colouring.
Cosmetic safety
Restricted cosmetic ingredient as IV/149 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: Anthocyanins (Cyanidin, Peonidin, Malvidin, Delphinidin, Petunidin, Pelargonidin).
Wording of conditions of use and warnings Purity criteria as set out in Commission Directive 95/45/EC (E163)
Health and Safety Considerations
Safety in Use
Anthocyanins (E163) are considered safe for use in food and cosmetics. They are not known to cause significant side effects and are generally well tolerated by the body. In addition, due to their antioxidant properties, they may offer health benefits by protecting cells from free radical damage.
Allergic Reactions
Allergic reactions to anthocyanins are extremely rare. Since they are extracted from natural sources such as fruits and vegetables, they are generally safe for human consumption and topical application.
Toxicity and Carcinogenicity
There is no evidence that anthocyanins are toxic or carcinogenic. On the contrary, they are known for their antioxidant and anti-inflammatory benefits, which may have positive health effects, including reducing the risk of chronic diseases.
Environmental and Safety Considerations
Anthocyanins are biodegradable and derived from renewable natural sources, making them a sustainable choice for use in food and cosmetic applications. Their environmental impact is minimal compared to synthetic colorants.
Regulatory Status
E163 (Anthocyanins) is approved as a food additive in the European Union and many other countries, including the United States. It is regulated for safe use in various applications, including food, cosmetics, and pharmaceuticals.
Studies
Daily intake is estimated at 500 mg to 1 gram (1).
Anthocyanins, which are used as a food coloring, are widely distributed in human diets, suggesting that we ingest large amounts of anthocyanins from plant-based foods. Mice were fed control, cyanidin 3-glucoside-rich purple corn color (PCC), high fat (HF) or HF + PCC diet for 12 wk. Dietary PCC significantly suppressed the HF diet-induced increase in body weight gain, and white and brown adipose tissue weights. Feeding the HF diet markedly induced hypertrophy of the adipocytes in the epididymal white adipose tissue compared with the control group. In contrast, the induction did not occur in the HF + PCC group. The HF diet induced hyperglycemia, hyperinsulinemia and hyperleptinemia. These perturbations were completely normalized in rats fed HF + PCC. An increase in the tumor necrosis factor (TNF)-alpha mRNA level occurred in the HF group and was normalized by dietary PCC. These results suggest that dietary PCC may ameliorate HF diet-induced insulin resistance in mice. PCC suppressed the mRNA levels of enzymes involved in fatty acid and triacylglycerol synthesis and lowered the sterol regulatory element binding protein-1 mRNA level in white adipose tissue. These down-regulations may contribute to triacylglycerol accumulation in white adipose tissue. Our findings provide a biochemical and nutritional basis for the use of PCC or anthocyanins as a functional food factor that may have benefits for the prevention of obesity and diabetes (2).
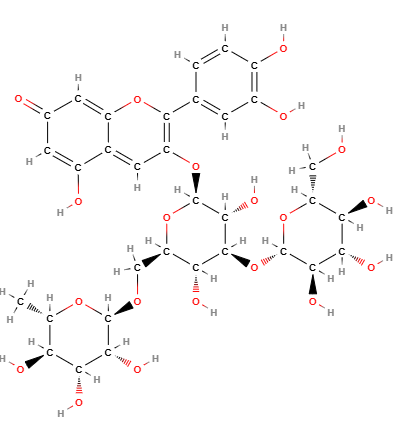 |  |
Molecular Formula: C15H11O+
Molecular Weight: 207.252 g/mol
CAS: 11029-12-2 39405-56-6 85763-44-6
EC Number: 600-954-3
Synonyms:
- Anthocyanidins
- 2-phenylchromenylium
- InChI=1/C15H11O/c1-2-6-12(7-3-1)15-11-10-13-8-4-5-9-14(13)16-15/h1-11H/q+
References_______________________________________________________________________
(1) Lila MA. Anthocyanins and Human Health: An In Vitro Investigative Approach. J Biomed_ Biotechnol. 2004;2004(5):306-313. doi: 10.1155/S111072430440401X.
(2) Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003


![]() E163
E163