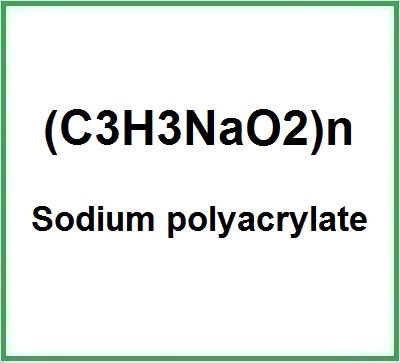
![]() Sodium polyacrylate
Sodium polyacrylate
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
8 pts from Nat45
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Sodium polyacrylate Review Consensus 8 by Nat45 (5724 pt) | 2023-Nov-22 16:42 |
| Read the full Tiiip | (Send your comment) |
Sodium polyacrylate is a high-molecular chemical compound, sodium salt of polyacrylic acid, a hydrophilic anionic polymer that is readily soluble in water, non-ionic.
The name describes the structure of the molecule:
- Sodium (Na). Sodium is an alkaline metal, a group of highly reactive elements found in group 1 of the periodic table. Sodium is soft and silvery-white in color. It is highly reactive, especially with water, and is commonly found in salts and minerals. It refers to the sodium (Na+) ions present in the compound.
- Polyacrylate refers to the polymer structure consisting of repeated units of acrylate, which is a carboxylate group attached to a vinyl group.
It occurs as a colourless, viscous liquid or as a fine, white powder.

The industrial sodium polyacrylate synthesis process has strict control of parameters such as temperature, pressure and reaction time. It also involves continuous monitoring and automated systems for adding reagents, maintaining conditions and purifying the product.
- Starting. Acrylic acid is fed into a stainless steel reactor equipped with a cooling system and mixed with water to form a solution.
- Neutralisation. Sodium hydroxide is added in a controlled manner to neutralise the solution. The neutralisation process is carefully monitored and adjusted to maintain the required pH. Dissolved oxygen, which can interfere with the polymerisation process, is removed after neutralisation by purifying the reactor with nitrogen gas
- Polymerisation. A free radical initiator, usually a peroxide or persulphate, is added to the reactor. The polymerisation process starts with heating as it is exothermic (generates heat) and the reactor cooling system is used to maintain a constant temperature, usually between 60° C and 70° C.
- Final stage. When polymerisation is complete, the contents of the reactor are transferred to a separate vessel for the precipitation and separation process. The polymer is separated, filtered, washed, dried and ground into a fine powder.
What it is used for and where it is used
Medicine
Sodium polyacrylate has shown antiulcerogenic activity mainly due to its action of covering the mucosa against gastric juice and is also an effective treatment against gastroesophageal reflux (1).
Food
Widely used in the food industry as a thickener.
Cosmetics
Used as surfactant and emulsifier, can be mixed with other surfactants with good dispersing properties. In the presence of acid dyes and cationic dyes or dispersed dyes and cationic dyes it is useful as an anti-precipitation agent.
These are its INCI functions in alphabetical order:
Absorbent. Absorbs substances dispersed or dissolved in aqueous solutions, water/oil, oil/water.
Binder agent. Ingredient that is used in cosmetic, food and pharmaceutical products as an anti-caking agent with the function of making the product in which it is incorporated silky, compact and homogenous. The binder, either natural such as mucilage, gums and starches or chemical, may be in the form of a powder or liquid.
Emulsion stabilizer. Emulsions are thermodynamically unstable. Emulsion stabilisers improve the formation and stability of single and double emulsions. It should be noted that in the structure-function relationship, molar mass plays an important role.
Film-forming agent. It produces a continuous ultra-thin film with an optimal balance of cohesion, adhesion and stickiness on the skin or hair to counteract or limit damage from external phenomena such as chemicals, UV rays and pollution.
Hair fixative. This ingredient has the ability to create, with its protective film, stiffness and hold in the hair, and also has the ability to form, with its hydrophilic and elastic properties, bonds between the hair fibres, to keep the hair in a particular shape for a certain time.
Skin conditioning agent. It is the mainstay of topical skin treatment by restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants.
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Viscosity control agent. It controls and adapts viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
Other uses
It is osmotically known to absorb 250 times its weight in water (2).
This super-absorbent can be found in disposable nappies, creams.
Used as a dispersant (recommended dosage 1-3%) for acid dyes and cationic dyes for dyeing wool and acrylic fibres.
Commercial applications
Cosmetic and Personal Care Industry. Used as a thickening and stabilizing agent in products like lotions, creams, and cleansers.
Textile Industry. Employed in diapers and other absorbent products for its ability to soak up large amounts of liquid.
Agriculture. Used as a water-absorbing gel to maintain moisture in soils and growing substrates.
Food Industry. Sometimes used as a food thickener.
Typical optimal commercial product characteristics Sodium acrilate liquid
| Appearance | Colourless, viscous liquid. |
| Boiling Point | 141ºC at 760 mmHg |
| Melting Point | >300 °C |
| Flash Point | Flash Point |
| PSA | 40.13000 |
| pH | 6.0-8.0 |
| Purity | 18±0.5 |
| Density (20℃)g/cm3 | 1.150 |
| Free monomer (CH2=CH-COOH) | % ≤0.5 |
| Chemical Safety |  |
 |  |
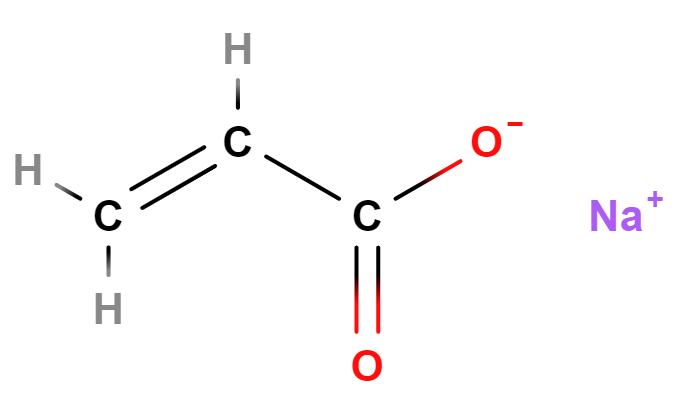 | 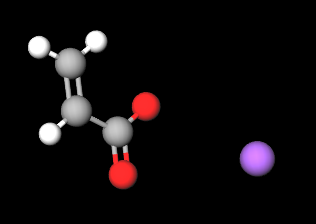 |
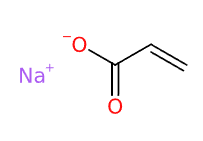
- Molecular Formula C3H3NaO2 (C3H3O2)n·Na C3H3NaO2)n/CH2=CH-COOH and CH2=CH-COONa
- Molecular Weight 94.045 g/mol
- Exact Mass 94.003075
- CAS: 7446-81-3 9003-04-7 25549-84-2 28603-11-4 95077-68-2
- DSSTox Substance ID DTXSID4027652 DTXSID0049783
- IUPAC sodium;prop-2-enoate
- InChI=1S/C3H4O2.Na/c1-2-3(4)5;/h2H,1H2,(H,4,5);/q;+1/p-1
- InChl Key NNMHYFLPFNGQFZ-UHFFFAOYSA-M
- SMILES C=CC(=O)[O-].[Na+]
- EC Number: 231-209-7 618-349-8 619-104-8 925-839-8
- UNII: 7C98FKB43H
- PubChem Substance ID 24868314
- MDL number MFCD00147949
- ISC 1429
Synonyms:
- Poly(acrylic acid sodium salt)
- 2-Propenoic acid, sodium salt
- sodium prop-2-enoate
- Sodium polyacrylate
- Sodium polyacrylate 4500
- Sodium polyacrylate solution
- Polyacrylic acid, sodium salt
- 7C98FKB43H
- CCRIS 3235
- Poly(acrylic acid sodium salt)
- Sodium poly acrylate
- Polyacrylate sodium salt
- Acrylic acid, sodium salt
- Poly(acrylic acid), sodium salt
- Acrylic acid polymer, sodium salt
- Acrylic acid, polymers, sodium salt
- 2-Propenoic acid, sodium salt (1:1)
- Propenoic acid, sodium carbonate polymer
- 2-Propenoic acid, sodium salt, homopolymer
- HSDB 6087
- Sodium 2-propenoate, homopolymer
- polyacrylate sodium
- 2-Propenoic acid, sodium salt (1:1), homopolymer
- Sodium 2-propenoate
- Polyco
- Acrysol lmw-45N
- Rhotex GS
- UNII-7C98FKB43H
- Hiviswako 105
References____________________________________________________________________
(1) Nakamura K, Ozawa Y, Furuta Y, Miyazaki H. Effects of sodium polyacrylate (PANa) on acute esophagitis by gastric juice in rats. Jpn J Pharmacol. 1982 Jun;32(3):445-56. doi: 10.1254/jjp.32.445.
Abstract. Sodium polyacrylate (PANa) is a water-soluble, high-molecular compound, and its aqueous solution shows a very high viscosity and stringiness. In the present study, preventive effects of PANa on three kinds of esophageal lesions induced by gastric juice were examined in comparison with those of aceglutamide aluminum and sodium alginate. The influences of PANa on gastric contents were also studied. The preventive effect of PANa given intraesophageally on esophageal lesions induced by the intraesophageal application of gastric juice was more potent than aceglutamide aluminum and sodium alginate. Oral administration of PANa inhibited the formation of esophageal ulcer by pylorus ligation more markedly than aceglutamide aluminum, whereas sodium alginate had no effect in a high dose of 500 mg/kg. In preventing gastric ulcer which occurred simultaneously with the esophageal ulcer after the pylorus ligation, aceglutamide aluminum was most potent, and PANa was as potent as sodium alginate. Oral administration of PANa showed a more protective effect than aceglutamide aluminum on the esophageal ulceration induced by the simultaneous ligations of the pylorus and limiting ridge, whereas sodium alginate in a high dose of 500 mg/kg had little effect on the ulcer formation. PANa caused only a slight increase in the pH of gastric juice and a slight decrease in pepsin activity. From the results, it may be concluded that PANa showed an antiulcerogenic activity mainly due to its mucosa covering action against gastric juice.
(2) Fan L, Zeng M, Zhou S, Lu L, Huang Y. Preliminary study of the relationship between water absorbency and zeta potentials of crosslinked poly(acrylic acid) J Control Release. 2011;152(Suppl):e260–e262. doi: 10.1016/j.jconrel.2011.09.050.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Sodium polyacrylate studies" about Sodium polyacrylate Review Consensus 8 by Nat45 (5724 pt) | 2019-May-08 22:50 |
| Read the full Tiiip | (Send your comment) |
Metal ions increase mechanical strength and barrier properties of collagen-sodium polyacrylate composite films.
Ma Y, Wang W, Wang Y, Guo Y, Duan S, Zhao K, Li S.
Int J Biol Macromol. 2018 Nov;119:15-22. doi: 10.1016/j.ijbiomac.2018.07.092. Epub 2018 Jul 17.
Characteristics of Sodium Polyacrylate/Nano-Sized Carbon Hydrogel for Biomedical Patch.
Park JK, Seo SK, Cho S, Kim HS, Lee CH.
J Nanosci Nanotechnol. 2018 Mar 1;18(3):1611-1614. doi: 10.1166/jnn.2018.15003.
An Intrinsically Self-Healing NiCo||Zn Rechargeable Battery with a Self-Healable Ferric-Ion-Crosslinking Sodium Polyacrylate Hydrogel Electrolyte.
Huang Y, Liu J, Wang J, Hu M, Mo F, Liang G, Zhi C
Angew Chem Int Ed Engl. 2018 Jul 26;57(31):9810-9813. doi: 10.1002/anie.201805618. Epub 2018 Jul 5.
Administration of intrapulmonary sodium polyacrylate to induce lung injury for the development of a porcine model of early acute respiratory distress syndrome.
Henderson WR, Barnbrook J, Dominelli PB, Griesdale DE, Arndt T, Molgat-Seon Y, Foster G, Ackland GL, Xu J, Ayas NT, Sheel AW.
Intensive Care Med Exp. 2014 Dec;2(1):5. doi: 10.1186/2197-425X-2-5. Epub 2014 Feb 26.
Sustainable and scalable production of monodisperse and highly uniform colloidal carbonaceous spheres using sodium polyacrylate as the dispersant.
Gong Y, Xie L, Li H, Wang Y.
Chem Commun (Camb). 2014 Oct 28;50(84):12633-6. doi: 10.1039/c4cc04998e.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (3)
Component type: Chemical Main substances: Last update: 2023-06-18 09:25:54 | Chemical Risk: |