Ethylhexyl triazone is a heterocyclic organic chemical compound known as Octyl Triazone or Uvinul T 150 and belongs to the class of triazines.
The name defines the structure of the molecule
- Ethylhexyl refers to the ethylhexyl group, which is a common structure in organic chemistry consisting of an 8-carbon alkyl chain with an ethyl group attached.
- Triazone refers to the specific chemical structure containing three nitrogen atoms in a ring formation, known as a triazone ring.
Description of raw materials used in production
Phthalic anhydride: Used as a raw material to synthesize the compound.
Isooctyl alcohol: Serves as the ethyl hexyl group.
Triazine: The core of the structure.
Step-by-step industrial chemical synthesis process
- Preparation of triazine: The triazine is prepared as the starting core.
- Synthesis of intermediate: Phthalic anhydride and isooctyl alcohol are reacted to form an intermediate.
- Combination with triazine: The intermediate is then combined with the triazine.
- Purification: The product is purified to remove any impurities.
- Quality control: A quality check is carried out to ensure that the product meets the required standards.
It appears in the form of a white powder.

What it is used for and where
Cosmetics
Ethylhexyl Triazone acts by absorbing and converting UVB radiation into less harmful energy, preventing it from reaching the skin. It acts as a UVB and UVA filter with broad-spectrum protection, is oil-soluble and used in cosmetic products at concentrations of up to 5%.
It is a restricted ingredient VI/15 as a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009.
As an INCI ingredient, it acts as follows:
UV filter. It is the defining ingredient in sun creams that can mitigate the sun's ultraviolet (UV) radiation, which is a high risk factor for the development of skin cancer, erythema and photo-ageing.
UV absorber. It acts by intercepting ultraviolet light before it can cause damage by reducing its energy through dissipation and returning it to a lower energy state.
Light stabilizer. It prevents light from degrading light-sensitive components and slows down degradation reactions that have already begun. The mechanism is, in a way, similar to antioxidants and the effectiveness depends on the.complexity of the formulation and the density of the product.
Commercial applications
Sun Care Products: It is mainly used in sun protection products such as sun lotions, creams, and sun sprays.
Skin Products: It may also be found in some facial creams and moisturizers with SPF, providing added protection against UVB rays.
Properties
UVB Absorption: Ethylhexyl Triazone is known for its ability to absorb UVB rays, which can cause sunburn and skin damage.
Stability: It has good stability, meaning it does not break down easily when exposed to the sun, maintaining its efficacy over time.
Solubility: It is soluble in oils, making it suitable for being formulated into a variety of skin products.
The most relevant studies on this chemical compound have been selected with a summary of their contents:
Ethylhexyl triazone studies

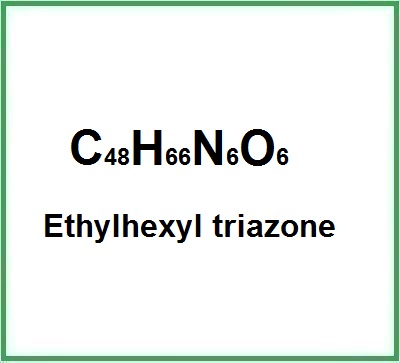
- Molecular Formula: C48H66N6O6
- Molecular Weight: 823.092 g/mol
- CAS: 88122-99-0 116244-12-3
- EC Number: 402-070-1 618-112-9
- PubChem Substance ID 329770240
- MDL number MFCD09753106
- Beilstein Registry Number 11001015
Synonyms:
- Octyl triazone
- Uvinul T 150
- Tris(2-ethylhexyl) 4,4',4''-((1,3,5-triazine-2,4,6-triyl)tris(azanediyl))tribenzoate
- Ethylhexyl Triazone(UV-T-150)
- Tris(2-ethylhexyl)-4,4',4''-(1,3,5-triazine-2,4,6-triyltriimino)tribenzoate
- 4,4',4''-(1,3,5-triazine-2,4,6-triyltriimino)trisbenzoic acid tris(2-ethylhexyl) ester
- 2-ethylhexyl 4-[[4,6-bis[4-(2-ethylhexoxycarbonyl)anilino]-1,3,5-triazin-2-yl]amino]benzoate
- Uvinul T150
- Octyltriazon
- Koptrizon
- AC1L4LEL
- EC 402-070-1
- SCHEMBL62195
- 116244-12-3
- UVT-150
- JGUMTYWKIBJSTN-UHFFFAOYSA-N
- BCP11409
- KS-00000I7S
- AKOS030228640
- ACN-053300
- DS-3287
- RL05489
- Benzoic acid, 4,4',4''-(1,3,5-triazine-2,4,6-triyltriimino)tris-, tris(2-ethylhexyl) ester
- Ethylhexyl triazone, analytical standard
- NCGC00183599-01
- AK683665
- AN-35356
- SC-19329
- HY-109655
- CS-0033785
- E1312
- FT-0688084
- 122T990
- Q-201502
- 2,4,6-Trianilino-P-(Carbo-2'-Ethylhexyl-1'-Oxy)-1,
- 2,4,6-Trianilino-P-(Carbo-2-Ethylhexyl-1-Oxy)-1,3,5-Triazine
- Ethylhexyl triazone, United States Pharmacopeia (USP) Reference Standard
- tris(2-ethylhexyl) 4,4',4''-(1,3,5-triazine-2,4,6-triyltriimino)trisbenzoate
- 2,4,6-trianilino-p-(carbo-2'-ethylhexyl-1'-oxy)-1,3,5-triazine
References_____________________________________________________________________
Seité, S., Zucchi, H., Duprat, L., Reygagne, P., Gomez, E., Rougier, A., et al. (2010). Efficacy and safety of a sunscreen containing 3% ethylhexyl triazone alone or in combination with 17% titanium dioxide: a randomized, double-blind, controlled clinical trial. Journal of the European Academy of Dermatology and Venereology, 24(2), 168-174.
Nohynek, G.J., Antignac, E., Re, T., Toutain, H. (2010). Safety assessment of personal care products/cosmetics and their ingredients. Toxicology and Applied Pharmacology, 243(2), 239-259.
Janjua, N.R., Mogensen, B., Andersson, A.M., Petersen, J.H., Henriksen, M., Skakkebaek, N.E., Wulf, H.C. (2004). Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. Journal of Investigative Dermatology, 123(1), 57-61.
Schlumpf, M., Kypke, K., Völkel, W., Krüger, T., Ma, R., Klotz, K., et al. (2008). Endocrine active UV filters: developmental toxicity and exposure through breast milk. Chimia, 62(5), 345-351.
García-Perdomo, H.A., Cely-Pérez, I., Almeyda-Arteta, M. (2019). Sunscreens: Updated on regulatory, instrumental, technological, and safety evaluation. Journal of Cosmetic Dermatology, 18(3), 724-731.
![]() Ethylhexyl triazone
Ethylhexyl triazone