![]() 1,2-hexanediol
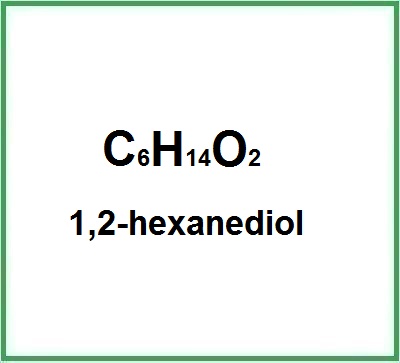
1,2-hexanediol
Rating : 7.3
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antimicrobial (1)23 pts from Frank123
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "1,2-hexanediol studies" about 1,2-hexanediol Review Consensus 24 by Frank123 (12058 pt) | 2020-Nov-09 11:49 |
| Read the full Tiiip | (Send your comment) |
The preserving agents that determine the shelf life of cosmetic products must have effective antimicrobial activity in compliance with safety requirements for topical use. Antimicrobial susceptibility tests showed that 1,2-hexanediol exhibited a broad spectrum activity against Gram-positive and Gram-negative bacteria with MIC of 0 · 5-2% (v / v) (1).

Regarding safety, type IV skin hypersensitivity reactions to 1,2-hexanediol (2) have not been observed.
Other studies
Dermal peptide delivery using enhancer molecules and colloidal carrier systems - part V#: Influence of enhancers on the permeation of PKEK through snake skin. Foerster A, Neubert RHH. Pharmazie. 2019 Mar 1;74(3):136-141. doi: 10.1691/ph.2019.8105.
Phototoxicity and chronic toxicity of methyl paraben and 1,2-hexanediol in Daphnia magna. Lee J, Park N, Kho Y, Lee K, Ji K. Ecotoxicology. 2017 Jan;26(1):81-89. doi: 10.1007/s10646-016-1743-6.
References__________________________________
(1) Food-grade antimicrobials potentiate the antibacterial activity of 1,2-hexanediol.
Yogiara, Hwang SJ, Park S, Hwang JK, Pan JG.
Lett Appl Microbiol. 2015 May;60(5):431-9. doi: 10.1111/lam.12398.
(2) Safety of a preservative system containing 1,2-hexanediol and caprylyl glycol.
Levy SB, Dulichan AM, Helman M.
Cutan Ocul Toxicol. 2009;28(1):23-4. doi: 10.1080/15569520802636082.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about 1,2-hexanediol Review Consensus 23 by Frank123 (12058 pt) | 2024-Oct-06 17:24 |
| Read the full Tiiip | (Send your comment) |
1,2-hexanediol is a chemical compound, alkanediol (alkanediols are composed of alkane and diol and are used as alternative antimicrobial preservatives for dermal formulations, hydrophilic creams, etc.) and has an amphiphilic structure, that is, it contains both a hydrophilic and a hydrophobic group. For this characteristic it has antimicrobial properties.
1,2-Hexanediol is an organic compound primarily used as a humectant, solvent, and preservative agent in cosmetics and personal care products. It is a diol, meaning it contains two hydroxyl (-OH) groups, which contribute to its moisturizing properties. It is commonly added to creams, lotions, serums, and cleansers to enhance texture and product stability, as well as to act as a secondary preservative.
Chemical Composition and Structure
1,2-Hexanediol is a bifunctional alcohol with the chemical formula C6H14O2. Its chemical structure includes a six-carbon chain with two hydroxyl (-OH) groups, which make it soluble in water and certain organic solvents. The hydroxyl groups contribute to its humectant properties, attracting and retaining moisture, making it useful in cosmetic formulations to keep skin hydrated.
Physical Properties
1,2-Hexanediol is a colorless, odorless, and viscous liquid that is easily soluble in water and alcohol. It has low volatility, making it stable in cosmetic formulations and helping to prevent moisture loss in water-based products. Due to its moisturizing properties and its ability to improve texture, 1,2-Hexanediol is a versatile ingredient in many cosmetic applications.
The name describes the structure of the molecule:
- "1,2-". These numbers specify the position of the alcohol groups on the carbon chain, in this case, on carbon atoms 1 and 2.
- "Hexane" refers to a six-carbon structure.
- "Diol". Indicates the presence of two alcohol (-OH) functional groups in the molecule.
Production Process
1,2-Hexanediol is synthetically produced through chemical reactions that involve the hydrogenation of aldehydes or the oxidation of alcohols. This process ensures a pure and safe product for use in cosmetic formulations.
Description of raw materials used in production:
- Adiponitrile. This organic compound serves as a key precursor. Typically, adiponitrile is produced by dimerizing butadiene, which involves the formation of a dinitrile.
- Hydrogen. Serves as the reducing agent in the reaction.
Step-by-step summary of industrial chemical synthesis process:
- Preparation of Adiponitrile. Butadiene is transformed into adiponitrile through a dimerization process. This process forms a structure with two nitrile (-CN) groups.
- Hydrogenation. Adiponitrile undergoes a hydrogenation reaction. During this phase, the nitrile groups of adiponitrile are reduced to alcohol (-OH) groups in the presence of a catalyst, usually nickel or cobalt-based, and hydrogen.
- Purification. Once the hydrogenation reaction is complete, the product contains 1,2-hexanediol along with other impurities. It's essential to purify the compound through vacuum distillation to get high-purity 1,2-hexanediol.
It appears as colorless liquid. Can be mixed with water, lower aliphatic hydrocarbons and fatty acids.

What it is used for and where
Cosmetics
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Solvent. It is the substance for dissolving or dispersing surfactants, oils, dyes, flavourings, bactericidal preservatives in solution.
It has antimicrobial activity and is included in cosmetic, personal care and hygiene product formulations as a preservative. As a solvent, it can dissolve or disperse surfactants, oils, dyes, aromas, bactericidal preservatives. It possesses permeability and good dispersing capacity for inorganic substances.
1,2-hexanediol tends to replace Parabens in the cosmetic industry. Parabens are used as antimicrobial preservatives in consumer products. Exposure to Methylparaben (MP) has been associated with negative health outcomes; therefore, 1,2-hexanediol has been introduced in the cosmetic field whose acute and chronic toxic effects are generally lower than those of Methylparaben but its effects on aquatic organisms should not be ignored (1).
Safety
The objective of this study was to investigate the percutaneous absorption of metronidazole (MTZ) in the topical formulations containing a combination of 1,4-cyclohexanediol and 1,2-hexanediol. Observations provide insight in formulating superior topical formulations with minimized potential systematic toxicity while maintaining therapeutic efficiency. A mechanistic explanation of the observed synergistic effect is proposed (3).
To study the potential for delayed Type IV dermal sensitivity of a new preservative system containing 1,2-hexanediol and caprylyl glycol, 200-subject repeat insult patch tests were performed with a 15% mixture of 1,2-hexanediol and caprylyl glycol (equal parts of the 2 ingredients) in carbomer gel and a cosmetic formulation at an actual use concentration. No delayed Type IV hypersensitivity reactions were observed (3).
Commercial Applications
Cosmetic Products. 1,2-Hexanediol is used as a humectant and solvent in cosmetics and skincare products.
Hair Products. Found in items such as shampoos and conditioners to enhance their texture.
Preserving Agents. It acts as a mild preservative in skincare products to prevent microbial growth.
Medical Applications
Dermatological Preparations. It can be found in some medicated creams and lotions due to its humectant properties.
Other uses
- Pesticides. Stabilizer
- Diesel engines. Antifreeze
- Automotive. Car brake fluid
- Paper. Printing ink.
- Wood. Preservative
- Medicine. Disinfectant
- Textile. Penetration agent
The most relevant studies on this chemical compound have been selected with a summary of their contents:
Typical optimal characteristics of 1,2-hexanediol as commercial product
| Density | 1.0±0.1 g/cm3 |
| Boiling Point | 223.5±0.0 °C a 760 mmHg |
| Melting Point | 45ºC |
| Flash Point | 95.8±13.0 °C |
| PSA | 40.46000 |
| LogP | 0.25 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Refraction Index | n20/D 1.442(lit.) |
| Shell life | 24 mesi |
 |  |
 |  |
- Linear Formula: CH3(CH2)3CH(OH)CH2OH
- Molecular Formula: C6H14O2
- Molecular Weight: 118.17
- Exact Mass 118.099380
- CAS: 6920-22-5
- EC Number: 230-029-6
- PubChem Substance ID 24852791
- MDL number MFCD00010737
- Beilstein Registry Number 1719244
- DSSTox Substance ID
- IUPAC hexane-1,2-diol
- InChI=1S/C6H14O2/c1-2-3-4-6(8)5-7/h6-8H,2-5H2,1H3
- InChl Key FHKSXSQHXQEMOK-UHFFFAOYSA-N
- SMILES CCCCC(CO)O
Synonyms:
- hexane-1,2-diol
- 1,2-hexane diol
- DL-1,2-Hexanediol
- dl-hexane-1,2-diol
- 1,2-Dihydroxyhexane
- 5,6-Dihydroxyhexane
- 1,2-Hexylene Glycol
- 2,2,6,6-Tetrakis(hydroxymEt)cyclohexanol
References_________________________________________________________________
(1) Lee J, Park N, Kho Y, Lee K, Ji K. Phototoxicity and chronic toxicity of methyl paraben and 1,2-hexanediol in Daphnia magna. Ecotoxicology. 2017 Jan;26(1):81-89. doi: 10.1007/s10646-016-1743-6.
Abstract. Parabens are used as antimicrobial preservatives in consumer products. Exposure to methylparaben (MP) has been associated with adverse health outcomes, therefore, an alternative compound, 1,2-hexanediol (1,2-H), has been applied for cosmetics. In the present study, the phototoxicity of MP and 1,2-H, as well as the toxic effect caused by chronic exposure, were investigated using Daphnia magna. The 48 h acute toxicity tests with D. magna were conducted under indoor or ultraviolet (UV) light irradiation conditions, i.e., exposure to 4 h/d sunlight. Changes in the transcription of genes related to oxidative stress were determined in D. magna juveniles, to investigate the underlying mechanism of phototoxicity. The 21 d chronic toxicity tests of MP and 1,2-H were performed under indoor light irradiation. Exposure to MP under environmental level of UV light was more detrimental to D. magna. Transcripts of catalase and glutathione-S-transferase genes in D. magna was significantly increased by co-exposure to MP and UV light. After 21 d of chronic exposure to MP and 1,2-H, the reproduction no-observed effect concentrations for D. magna were 1 and >10 mg/L, respectively. The present study showed that exposure to UV could magnify the toxicity of MP on daphnids. Although acute and chronic toxicities of 1,2-H were generally lower than those of MP, its effects on other aquatic organisms should not be ignored. Further studies are needed to identify other mechanisms of MP phototoxicity.
(2) Li N, Jia W, Zhang Y, Tan F, Zhang J. Synergistic effect of 1,4-cyclohexanediol and 1,2-hexanediol on percutaneous absorption and penetration of metronidazole. Int J Pharm. 2011 Aug 30;415(1-2):169-74. doi: 10.1016/j.ijpharm.2011.05.069.
(3) Levy SB, Dulichan AM, Helman M. Safety of a preservative system containing 1,2-hexanediol and caprylyl glycol. Cutan Ocul Toxicol. 2009;28(1):23-4. doi: 10.1080/15569520802636082.
Abstract. To study the potential for delayed Type IV dermal sensitivity of a new preservative system containing 1,2-hexanediol and caprylyl glycol, 200-subject repeat insult patch tests were performed with a 15% mixture of 1,2-hexanediol and caprylyl glycol (equal parts of the 2 ingredients) in carbomer gel and a cosmetic formulation at an actual use concentration. No delayed Type IV hypersensitivity reactions were observed.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances:
Last update: 2021-12-05 12:51:55 | Chemical Risk: |