![]() Ethylhexyl palmitate
Ethylhexyl palmitate
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from AColumn
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Ethylhexyl palmitate studies" about Ethylhexyl palmitate Review Consensus 10 by AColumn (9336 pt) | 2022-Sep-13 12:00 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Albro, P. W., & Thomas, R. O. (1973). Enzymatic hydrolysis of di-(2-ethylhexyl) phthalate by lipases. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism, 306(3), 380-390.
Abstract. Di-(2-ethylhexyl) phthalate was hydrolyzed by lipases from a variety of rat tissues. Of the preparations studied, only glycerol-ester hydrolase (pancreatic lipase, EC 3.1.1.3) and sterol-ester -hydrolase (cholesteryl ester hydrolase, EC 3.1.1.13) were unable to hydrolyze the phthalate diester. However, out of 15 different tissue preparations, only the liver alkaline lipase preparation could hydrolyze mono-(2-ethylhexyl) phthalate at a significant rate....
Gawas SD, Rathod VK. Ultrasound Assisted Green Synthesis of 2-Ethylhexyl Stearate: A Cosmetic Bio-lubricant. J Oleo Sci. 2020 Sep 2;69(9):1043-1049. doi: 10.5650/jos.ess19322.
Abstract. The 2-ethylhexyl stearate is used as a bio-lubricant in various cosmetic products. The present study is focused on the biocatalyzed esterification of 2-ethylhexanol and stearic acid to form 2-ethylhexyl stearate catalyzed by Fermase CALB 10000 in the presence of ultrasound treatment. The maximum conversion (95.87%) was obtained at molar ratio of 2-ethylhexanol to stearic acid 2:1, enzyme amount of 2 % (w/w), power 80 W, duty cycle 50 % and temperature 50°C in comparatively short reaction time (3 h) in the presence of Fermase as a catalyst. At optimum conditions, it is observed that in the presence of ultrasound; the reaction time minimizes up to 4 h as compared to mechanical stirring method (7 h). The physiochemical properties for the 2-ethylhexyl palmitate were also evaluated.
Richetti A, Leite SG, Antunes OA, Lerin LA, Dallago RM, Emmerich D, Di Luccio M, Vladimir Oliveira J, Treichel H, de Oliveira D. Assessment of process variables on 2-ethylhexyl palmitate production using Novozym 435 as catalyst in a solvent-free system. Bioprocess Biosyst Eng. 2010 Mar;33(3):331-7. doi: 10.1007/s00449-009-0328-7.
Abstract. This work reports the optimization of 2-ethylhexyl palmitate production by esterification reaction in a solvent-free system using a commercial lipase as catalyst. For this, a sequential strategy was performed applying three experimental designs. An empirical model was built so as to assess the effects of process variables on the reaction conversion. Afterward, the operating conditions that optimized 2-ethylhexyl palmitate production were determined to be acid to alcohol molar ratio of 1:5.5, 70 degrees C, 150 rpm and 10.5 wt% of enzyme, leading to a reaction conversion as high as 93%. From this point, a kinetic study was carried out evaluating the influence of acid to alcohol molar ratio, enzyme concentration and temperature on product yield. Results obtained in this step allow to conclude that an excess of alcohol (acid to alcohol molar ratio of 1:6), relatively low enzyme concentration (10 wt%) and temperature of 70 degrees C led to nearly complete reaction conversion.
Xu, J., Zhang, R., Liu, C., Yu, Y., Wang, F., & Deng, L. (2021). High Efficient Biosynthesis 2-Ethylhexyl Palmitate in a Rotating Packed Bed Reactor. Applied biochemistry and biotechnology, 193(8), 2420-2429.
Abstract. 2-Ethylhexyl palmitate (2-EHP) is one of the important chemical products. Normally, 2-EHP is produced through the esterification. Since 2-EHP has a high viscosity, the mass transfer is significantly influenced with the product accumulation. In this work, a rotating packed bed reactor with intensive mixing was employed to solve the problem in the mass transfer during the enzymatic reaction. Under the optimal conditions, compared with the traditional continuous stirred-tank reactor (CSTR), the RPB reactor enhanced the final yield of 2-EHP, and shortened the reaction time to 1 h. In addition, the enzyme has a longer life-time in the RPB reactor, with production yield of closing to 99% after 9 batches. The results of this research indicated that the RPB has a great potential to be applied in the enzymatic production of 2-EHP.
Albro, P. W., & Thomas, R. O. (1973). Enzymatic hydrolysis of di-(2-ethylhexyl) phthalate by lipases. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism, 306(3), 380-390.
Abstract. Di-(2-ethylhexyl) phthalate was hydrolyzed by lipases from a variety of rat tissues. Of the preparations studied, only glycerol-ester hydrolase (pancreatic lipase, EC 3.1.1.3) and sterol-ester -hydrolase (cholesteryl ester hydrolase, EC 3.1.1.13) were unable to hydrolyze the phthalate diester. However, out of 15 different tissue preparations, only the liver alkaline lipase preparation could hydrolyze mono-(2-ethylhexyl) phthalate at a significant rate.
He, X. L., Chen, B. Q., & Tan, T. W. (2002). Enzymatic synthesis of 2-ethylhexyl esters of fatty acids by immobilized lipase from Candida sp. 99–125. Journal of Molecular Catalysis B: Enzymatic, 18(4-6), 333-339.
Abstract. The 2-ethylhexyl esters of fatty acids were synthesized by immobilized lipase from Candida sp. 99–125. The reuse stability of immobilized lipase was at least four batches. The conditions of enzymatic synthesis of 2-ethylhexyl palmitate were optimized. In the system of petroleum ether, 10% (w/w) immobilized lipase was used in the esterfication of 2-ethyl hexanol (7.8 mmol) and palmitic acid (7.8 mmol) at 40 °C with silica gel as the water absorbent. The esterification degree was 91% under these conditions. The purity of 2-ethylhexyl palmitate was 98% after purification consisting washing by water and evaporation to remove the organic solvent.
Choi, S., Kim, B. H., Yoon, S. W., Lee, M. W., Im, D. J., & Kim, I. H. (2022). Lipase-catalyzed synthesis of 2-ethylhexyl palmitate in a solvent free system using step changes in temperature. Biochemical Engineering Journal, 177, 108261.
Abstract. Lipase-catalyzed synthesis of 2-ethylhexyl palmitate (2-EHP) from 2-ethylhexyl alcohol and palmitic acid was carried out in a solvent-free system. A commercial liquid lipase (Eversa Transform 2.0, Novozymes) from Thermomyces lanuginosus was immobilized on Lewatit VP OC 1600, a macroporous hydrophobic carrier. The efficacy of this Eversa immobilized lipase prepared in this study was evaluated on the synthesis of 2-EHP compared with that of Novozym 435 (from Candida antarctica), Lipozyme RM IM (from Rhizomucor miehei), Lipozyme TL IM (from Thermomyces lanuginosus), and the liquid Eversa lipase (commercial name: Eversa Transform 2.0). Among these lipases, the Eversa immobilized lipase was the most effective for the synthesis of 2-EHP. Optimum conditions for the synthesis of 2-EHP using this enzyme were a temperature of 55 °C and enzyme loading of 2% (based on the total weight of substrate). The conversion of 97% was achieved under these optimum conditions. Finally, as a cost-saving strategy, a step change in the reaction temperature was introduced. When a step change in the reaction temperature between 55 °C and 45 °C used, an identical degree of conversion was achieved compared to a constant reaction temperature of 55 °C throughout the reaction.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Ethylhexyl palmitate Review Consensus 10 by AColumn (9336 pt) | 2023-Jul-28 16:34 |
| Read the full Tiiip | (Send your comment) |
Ethylhexyl palmitate or 2-Ethylhexyl palmitate is a chemical compound, an ester that is obtained by synthesis resulting from the reaction of 2-ethylhexyl alcohol with palmitic acid.
The name defines the structure of the molecule:
- Ethylhexyl refers to a specific chain derived from the combination of hexyl (a six-carbon chain) and ethyl (a two-carbon chain).
- Palmitate refers to the form of ester or salt of palmitic acid, a type of saturated fatty acid commonly found in both animals and plants, especially palm oil.
The synthesis process takes place in several stages:
- Preparation. The process begins with the preparation of acid and alcohol. Palmitic acid is a common fatty acid found in fats and waxes, and 2-ethylhexanol is a fatty alcohol. Both are commercially available and are prepared for reaction.
- Esterification. Palmitic acid and 2-ethylhexanol are inserted into a reaction vessel. An acid catalyst, often sulfuric acid, is added to the mixture which, when heated, starts the esterification reaction. This reaction causes the formation of Ethylhexyl Palmitatee water.
- Separation. The reaction mixture is allowed to cool. Ethylhexyl Palmitate, being less polar than water, will separate. Water and all non-reacted materials can be removed.
- Purification. Ethylhexyl Palmitate is purified by distillation, heated and the vapors are collected and condensed. This process helps to remove any remaining impurities.
- Quality control. The final product is tested to ensure it meets the specifications required for use in cosmetics and personal care products. This includes checking its purity, color and smell.
It appears as an odourless, colourless liquid or as a white, poorly hydrolysable, hardly oxidisable powder.


What it is used for and where
Raw material for cosmetics
Cosmetics
It is a dry, yet breathable oil with the ability to penetrate and moisturise the skin, and is thermally stable.
It is used in cosmetics in creams, skin care products, foundations, skin care oils, mascaras, hair oils, lipsticks and in other body care products as an emollient, emulsifier. It provides a smooth and soft skin feel without greasiness with good permeability and is non-sticky. It is a good substitute for mineral oil and is compatible with other emollients.
It also has a moisturising, softening, dispersing function. It has good biodegradability and preservation.
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Fragrance. It plays a very important role in the formulation of cosmetic products as it allows perfume to be enhanced, masked or added to the final product, improving its commercial viability. The consumer always expects to find a pleasant scent in a cosmetic product.
Applications
- Skin Conditioning Agent - Helps keep the skin soft and moisturized, reducing dryness.
- Solvent - Can be used as a solvent for active ingredients or colorants in cosmetic formulations.
- Oil Substitute - Sometimes used as a lightweight, non-greasy alternative to oils in various products.
- Perfume Base - Its volatility makes it suitable as a base in perfumes.
Studies:
2-Ethylhexyl palmitate was prepared in organic solvents catalysed by an immobilised lipase QLM. Microwave irradiation was used to enhance the activity of the enzyme and shorten the reaction time. Microwave reaction conditions were optimised. Compared to the free QLM under classical heating, the immobilised QLM under microwave oven showed higher enzyme activity and the conversion could reach 99% in about 3.0 hours. Furthermore, the immobilised QLM showed excellent reusability under microwave irradiation (1).
This work reports on the application of a lipase in the esterification of 2-Ethylhexyl palmitate in a solvent-free system with an immobilised lipase (Lipozyme RM IM). A sequential strategy was used by applying two experimental designs to optimise the production of 2-ethylhexyl palmitate. An empirical model was then constructed to evaluate the effects of process variables on reaction conversion. Subsequently, the operating conditions that optimise the production of 2-ethylhexyl palmitate were established as an acid/alcohol molar ratio of 1: 3, temperature of 70 degrees C, stirring speed of 150 rpm, 10% by weight of enzyme, leading to a reaction conversion of 95%. From this point, a kinetic study was conducted to evaluate the effect of the acid: alcohol molar ratio, enzyme concentration and temperature on product conversion. The results obtained at this stage show that an excess of alcohol (molar ratio of acid to alcohol of 1: 6), relatively low enzyme concentration (10% by weight) and a temperature of 70 degrees C lead to conversions close to 100% (2).
For more information:
| Appearance | White powder or colorless liquid |
| Boiling Point | 407.2ºC at 760 mmHg |
| Melting Point | 2°C |
| Flash Point | 203.7ºC |
| Density | 0.86 g/cm3 |
| PSA | 26.30000 |
| Reflexion Index | 1.449 |
| Vapor Pressure | 7.7E-07mmHg at 25°C |
| LogP | 8.22730 |
| Acid value | ≤0.5 (mgKOH/g) |
| Water | ≤0.5% |
| Shelf life | 2 years |
 |  |
 |  |
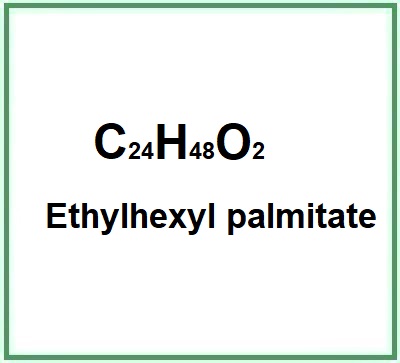
- Molecular Formula C24H48O2
- Molecular Weight 368.64
- Exact Mass 368.36500
- CAS 29806-73-3
- UNII 2865993309
- EC Number 249-862-1
- DSSTox Substance ID
- IUPAC octan-3-yl hexadecanoate
- InChI=1S/C24H48O2/c1-4-7-9-10-11-12-13-14-15-16-17-18-20-22-24(25)26-23(6-3)21-19-8-5-2/h23H,4-22H2,1-3H3
- InChl Key GJQLBGWSDGMZKM-UHFFFAOYSA-N
- SMILES CCCCCCCCCCCCCCCC(=O)OC(CC)CCCCC
- MDL number MFCD00072255
- PubChem Substance ID
- RTECS RT4740000
Synonyms
- Palmitic acid, 2-ethylhexyl ester
- Palmitic acid 2-ethylhexyl ester
- 2-Ethylhexyl palmitate
- Hexadecanoic acid, 2-ethylhexyl ester
- 2-Ethylhexyl hexadecanoate
- Wickenol 155
References_____________________________________________________________________
(1) Wang L, Zhang Y, Zhang Y, Zheng L, Huang H, Wang Z. Synthesis of 2-Ethylhexyl Palmitate Catalyzed by Enzyme Under Microwave. Appl Biochem Biotechnol. 2018 May;185(1):347-356. doi: 10.1007/s12010-017-2666-2.
(2) Richetti A, Leite SG, Antunes OA, de Souza AL, Lerin LA, Dallago RM, Paroul N, Di Luccio M, Oliveira JV, Treichel H, de Oliveira D. Optimization of 2-ethylhexyl palmitate production using lipozyme RM IM as catalyst in a solvent-free system. Appl Biochem Biotechnol. 2010 Apr;160(8):2498-508. doi: 10.1007/s12010-009-8756-z.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Natural Main substances:
Last update: 2017-06-16 10:46:23 | Chemical Risk: |