EDTA, or Ethylenediaminetetraacetic Acid, is a synthetic amino acid and a chelating agent widely used in various industries, including cosmetics, pharmaceuticals, and food. In cosmetic formulations, EDTA is valued for its ability to bind metal ions, thus preventing them from interfering with the stability and effectiveness of the product. This property makes EDTA an essential ingredient in formulations aimed at enhancing the longevity and performance of active ingredients.
Chemical Composition and Structure
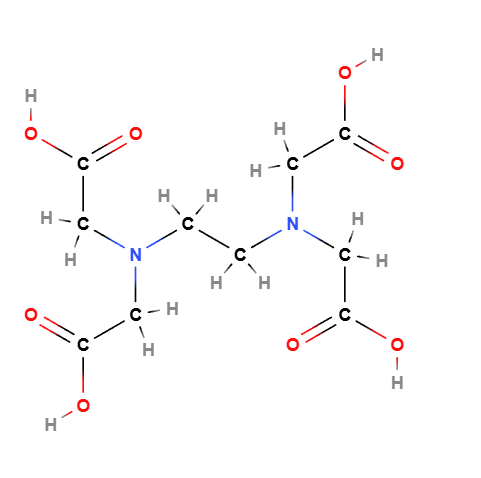
- Chemical Formula: C10H16N2O8
- Structure: Consists of an ethylene backbone with four acetic acid functional groups attached, allowing it to effectively bind metal ions.
The unique structure of EDTA allows it to form stable complexes with a wide range of metal ions, thus enhancing the overall stability of cosmetic products.
Physical Properties
Appearance: Typically a white crystalline powder.
Solubility: Soluble in water; insoluble in organic solvents.
pH: Generally neutral to slightly acidic when dissolved in water.
Odor: Odorless.
Stability: Stable under normal storage conditions; should be protected from excessive heat and light.
Production Process
Synthesis: EDTA is produced through the reaction of ethylenediamine with chloroacetic acid in a controlled environment, resulting in the formation of EDTA.
Purification: The resulting product is purified to remove any unreacted materials and by-products, ensuring a high-quality chelating agent.
Formulation: Purified EDTA is incorporated into various cosmetic products to enhance their stability and effectiveness.
Applications
Cosmetics: Commonly found in shampoos, conditioners, and skincare products to improve product stability and enhance the performance of active ingredients by preventing metal ion interference.
Pharmaceuticals: Used in formulations to stabilize drugs and enhance their efficacy.
Food Industry: Employed as a preservative and stabilizer in various food products to maintain quality and freshness.
Environmental and Safety Considerations
EDTA is generally regarded as safe for use in cosmetics when applied according to recommended guidelines.
EDTA is generally considered safe for use in cosmetics when applied according to recommended guidelines, however, as a chelating agent it is shown to be cytotoxic and weakly genotoxic but not carcinogenic. Concern concerns cosmetic formulations which may be inhaled (1).
Cases of contact allergy to EDTA have been reported (2)
However, concerns regarding its environmental impact have been raised due to its persistence in aquatic environments.
Responsible sourcing and formulation practices are essential to ensure that the ingredient is used sustainably and does not contribute to environmental harm.
Molecular Formula C10H16N2O8
Molecular Weight 292.24 g/mol
CAS 60-00-4
UNII 9G34HU7RV0
EC Number 200-449-4
DTXSID6022977
Synonyms:
EDTA
Edetic acid
References__________________________________________________________________________
(1) Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21 Suppl 2:95-142. doi: 10.1080/10915810290096522. PMID: 12396676.
Abstract. EDTA (ethylenediamine tetraacetic acid) and its salts are substituted diamines. HEDTA (hydroxyethyl ethylenediamine triacetic acid) and its trisodium salt are substituted amines. These ingredients function as chelating agents in cosmetic formulations. The typical concentration of use of EDTA is less than 2%, with the other salts in current use at even lower concentrations. The lowest dose reported to cause a toxic effect in animals was 750 mg/kg/day. These chelating agents are cytotoxic and weakly genotoxic, but not carcinogenic. Oral exposures to EDTA produced adverse reproductive and developmental effects in animals. Clinical tests reported no absorption of an EDTA salt through the skin. These ingredients are likely, however, to affect the passage of other chemicals into the skin because they will chelate calcium. Exposure to EDTA in most cosmetic formulations, therefore, would produce systemic exposure levels well below those seen to be toxic in oral dosing studies. Exposure to EDTA in cosmetic formulations that may be inhaled, however, was a concern. An exposure assessment done using conservative assumptions predicted that the maximum EDTA dose via inhalation of an aerosolized cosmetic formulation is below that shown to produce reproductive or developmental toxicity. Because of the potential to increase the penetration of other chemicals, formulators should continue to be aware of this when combining these ingredients with ingredients that previously have been determined to be safe, primarily because they were not significantly absorbed. Based on the available data, the Cosmetic Ingredient Review Expert Panel found that these ingredients are safe as used in cosmetic formulations.
(2) de Groot AC. Contact allergy to EDTA in a topical corticosteroid preparation. Contact Dermatitis. 1986 Oct;15(4):250-2. doi: 10.1111/j.1600-0536.1986.tb01352.x.
Raymond, J. Z., & Gross, P. R. (1969). EDTA: preservative dermatitis. Archives of Dermatology, 100(4), 436-440.
Abstract. Ethylenediamine tetraacetate is a widely used preservative, especially in ophthalmic solutions. It was found to be the cause of an acute allergic conjunctivitis and periorbital dermatitis. Subsequent testing revealed two additional cases of delayed hypersensitivity to this compound. Two of the three cases cross-reacted to ethylenediamine, a structurally related compound. Recommendation for patch testing with 1% concentration in water or petrolatum is made. The increasing problem of reactions to preservatives is discussed.
![]() EDTA
EDTA