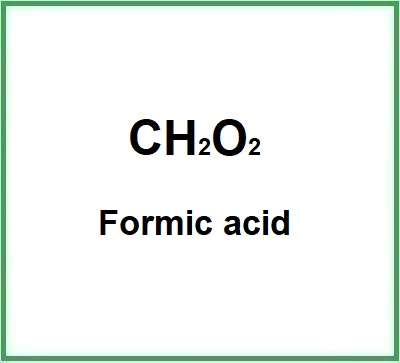
Formic acid is one of the highest value-added chemicals, which has a broad market and wide application range in dyeing, food additives, pharmaceutical and cosmetic industries .
Formic acid is a weak acid produced by Clostridium acetobutylicum, a bacterium of the family Clostridiaceae.
The name describes the structure of the molecule:
- 'formic'. This term is derived from the Latin word 'formica', meaning ant. The name is derived from the fact that formic acid was first isolated from ants.
- 'Acid' refers to its acidic properties, as formic acid is a corrosive and strong acid.
The synthesis process takes place in different steps:
- Catalytic oxidation. Methanol (CH3OH) is oxidised using a catalyst, usually a metal catalyst such as platinum (Pt) or palladium (Pd). The reaction takes place under controlled conditions, often with the addition of oxygen (O2) or air.
- Formation of intermediates. The oxidation of methanol leads to the formation of formaldehyde (HCHO) as an intermediate product. This reaction is exothermic and typically occurs at high temperatures.
- Further oxidation. The formaldehyde produced in the previous step is further oxidised to form form formic acid (HCOOH). This oxidation process usually requires additional oxidising agents such as oxygen or other oxidising agents such as nitric acid (HNO3) or chromic acid (H2CrO4).
- Separation and purification. Once formed, the formic acid must be separated from the reaction mixture and purified. This is typically done through techniques such as distillation or extraction. Distillation involves heating the mixture to vaporise the formic acid, which is then condensed and collected as a pure liquid.
It is worth noting that there are alternative synthesis routes for formic acid, including hydrolysis of hydrogen cyanide (HCN) or carbon dioxide (CO2). In addition, formic acid can also be obtained from natural sources, such as certain plants and insects that produce it as a defence mechanism.
Formic acid (methanoic acid) is a weak acid capable of causing oxidative stress. Low concentrations of formic acid are widely used as a major ingredient of antiseptics. On the other hand, formic acid has been shown to be a toxic metabolite of methanol, it is a commonly used organic solvent that has been long known to be a selective human neurotoxin (1).
Formic acid in the human body.
Human skin fibroblasts in culture can oxidize beta-methyl fatty acids, such as phytanic acid and 3-methylhexadecanoic acid, to CO2 and water-soluble products. The latter are released largely into the culture medium. The major water-soluble product formed from [1-14C]phytanic and [1-14C]3-methylhexadecanoic acids is [14C]formic acid. As phytanic acid and 3-methylhexadecanoic acids contain beta-methyl groups and theoretically cannot be degraded by beta-oxidation, we postulate that formic acid is formed from fatty acids by alpha-oxidation. The marked reduction in formic acid production from beta-methyl fatty acids in peroxisome-deficient skin fibroblasts suggests that peroxisomes are involved in the generation of C1 units (2).
What it is used for and where
Industrially it appears as a colourless transparent liquid.

Food
Ingredient listed in the European food additives list as E236, preservative.
Formic acid in animal nutrition.
The treatment of feed given to laying hens with 0.5% formic acid reduced significantly the isolation rate of salmonellas and was associated with a reduction in the incidence of infection in newly hatched chicks. These improvements were not sustained until slaughter, however, as growing birds acquired salmonellas, probably from feed which was not acid treated. The data indicate that formic acid treatment of chicken food could have important benefits for the public health (3).
Cosmetics
Fragrance. It plays a decisive and important role in the formulation of cosmetic products as it provides the possibility of enhancing, masking or adding fragrance to the final product, increasing its marketability. The consumer always expects to find a pleasant or distinctive scent in a cosmetic product.
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
pH adjuster. This ingredient tends to restore the pH of a cosmetic formulation to its optimal value. The correct pH value is an essential determinant for lipid synthesis in the stratum corneum. The average physiological pH value of the face ranges between 5.67 and 5.76. The hair fibre has a pH value of 3.67.
Safety of Formic acid in cosmetics.
Formic acid functions as a fragrance ingredient, preservative, and pH adjuster in cosmetic products, whereas sodium formate functions as a preservative. Because of its acidic properties, formic acid is a dermal and ocular irritant. However, when used as a pH adjuster in cosmetic formulations, formic acid will be neutralized to yield formate salts, for example, sodium formate, thus minimizing safety concerns. Formic acid and sodium formate have been used at concentrations up to 0.2% and 0.34%, respectively, with hair care products accounting for the highest use concentrations of both ingredients. The low use concentrations of these ingredients in leave-on products and uses in rinse-off products minimize concerns relating to skin/ocular irritation or respiratory irritation potential. The Cosmetic Ingredient Review Expert Panel concluded that formic acid and sodium formate are safe in the present practices of use and concentration in cosmetics, when formulated to be nonirritating (4).
Formic acid as an ecological agent
Formic acid was used for the nitrate reduction as a reductant in the presence of Pd:Cu/gamma-alumina catalysts. Negligible amount of ammonia was detected, and no nitrite was detected, possibly due to buffering effect of bicarbonate that is in situ produced by the decomposition of formic acid, and due to the sustained release of H2 gas (5).

- Molecular formula CH2O2
- Molecular weight 46,03
- CAS 64-18-6
- UNII 0YIW783RG1
- EC Number 200-579-1
- DSSTox ID DTXSID2024115
- IUPAC formic acid
- InChl=1S/CH2O2/c2-1-3/h1H,(H,2,3)
- InChl Key BDAGIHXWWSANSR-UHFFFAOYSA-N
- SMILES C(=O)O
- MDL number MFCD00003297
- PubChem Substance ID 329799662
- ChEBI 30751
- Beilstein 1209246
- RTECS LQ4900000
- JECFA 79
- NACRES NA.21
- FEMA 2407
- ICSC 0485
- Nikkaji J1.402H
- NCI C83719
Synonyms:
- Bilorin
- Formira
- Add-F
- Amasil
References___________________________________________________________________
(1) Formic acid induces Yca1p-independent apoptosis-like cell death in the yeast Saccharomyces cerevisiae.
Du L, Su Y, Sun D, Zhu W, Wang J, Zhuang X, Zhou S, Lu Y.
FEMS Yeast Res. 2008 Jun;8(4):531-9. doi: 10.1111/j.1567-1364.2008.00375.x. Epub 2008 Apr 29.
(2) Formic acid is a product of the alpha-oxidation of fatty acids by human skin fibroblasts: deficiency of formic acid production in peroxisome-deficient fibroblasts.
Poulos A, Sharp P, Singh H, Johnson DW, Carey WF, Easton C. - Biochem J. 1993 Jun 1;292 ( Pt 2):457-61.
(3) The vertical transmission of salmonellas and formic acid treatment of chicken feed. A possible strategy for control. Humphrey TJ, Lanning DG. Epidemiol Infect. 1988 Feb;100(1):43-9.
(4) Safety Assessment of Formic Acid and Sodium Formate as Used in Cosmetics.
Johnson W Jr, Heldreth B, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA.
Int J Toxicol. 2016 Nov;35(2 suppl):41S-54S. Review.
(5) Formic acid as an alternative reducing agent for the catalytic nitrate reduction in aqueous media.
Choi EK, Park KH, Lee HB, Cho M, Ahn S.
J Environ Sci (China). 2013 Aug 1;25(8):1696-702.
![]() Formic Acid
Formic Acid