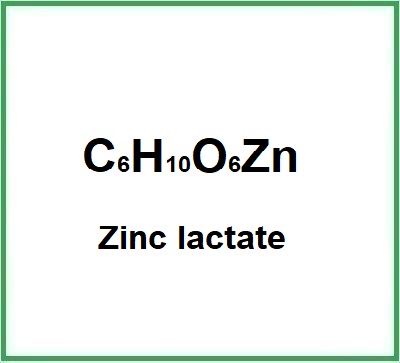
Zinc lactate is a chemical compound formed from the salt of lactic acid and zinc.
The name defines the structure of the molecule:
- "zinc" is a chemical element with the symbol Zn and the atomic number 30.
- "Lactate" is a compound produced when the body breaks down carbohydrates to use them for energy purposes in periods of low oxygen levels.
The synthesis process takes place in several stages.
- The first phase involves the reaction of lactic acid with zinc carbonate or zinc hydroxide to produce zinc lactate and water or carbon dioxide.
- The resulting mixture is then filtered to remove any unreacted zinc or hydroxide carbonate.
- The filtrate is evaporated at reduced pressure to obtain zinc lactate crystals.
- The crystals are collected and dried to obtain the final product, zinc lactate.
It appears in the form of a white powder

What it is used for and where
Cosmetics
It is a restricted ingredient as III/24 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Regulated by 82/368/EEC. Maximum concentration in ready for use preparation 1% (as zinc). Risk: Water-soluble zinc salts with the exception of zinc 4- hydroxy-benzenesulphonate (entry 25) and zinc pyrithione (Annex II, entry 1670)
- Deodorant agent. When substances that give off an unpleasant odour are included in cosmetic formulations (typical examples are methyl mercaptan and hydrogen sulphide derived from garlic), deodorants attenuate or eliminate the unpleasant exhalation. It helps counteract the formation of bad odours on body surfaces.
- Zinc lactate is also used in some cosmetic products for its astringent properties. It can help tighten the skin and minimise pores, making it useful in products for oily or acne-prone skin.
- Oral hygiene products. In the oral hygiene sector, zinc lactate is used in toothpastes, mouthwashes and other oral hygiene products due to its antimicrobial properties. It can help prevent dental plaque and reduce bad breath.
Food
Zinc lactate is often used as a food supplement and food additive. It serves as a source of zinc, a necessary mineral that supports various biological acts, including immune function, protein synthesis, DNA synthesis and cell division. It is also used as a flavour enhancer in some foods.
Medical
Zinc lactate is used in some medicinal products for its ability to heal wounds. It can help heal minor cuts, burns and other skin irritations.
Animal feeding
It is also used as a zinc supplement in animal feed to provide livestock with the necessary amount of this essential mineral.
Studies
Zinc is an essential trace element for all eukaryotic organisms. It is required for the catalytic activity or the structural integrity of more than 300 enzymes (1).
Zinc lactate is a salt of lactic acid, which is a major fermentative product of lactic acid bacteria. Lactates are used as food preservatives. Several mechanisms are responsible for the antimicrobial properties of lactic acid and its salts (2).
Zinc lactate has been used to reduce some respiratory tract infections.
Zinc and aluminum lactate, as well as zinc and aluminum chloride (0.1%), worked synergistically with 100 IU of nisin per ml to control the growth of L. monocytogenes Scott A.(4).
Zinc lactate is used in toothpastes as an antibacterial (5).
- Molecular formula C6H10O6Zn
- Molecular weight 243.53
- CAS 16039-53-5
- EC number 240-178-9
Synonyms:
References_______________________________________________________________________
(1) Berg JM, Shi Y The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996 Feb 23; 271(5252):1081-5.
Abstract. Zinc ions are key structural components of a large number of proteins. The binding of zinc stabilizes the folded conformations of domains so that they may facilitate interactions between the proteins and other macromolecules such as DNA. The modular nature of some of these zinc-containing proteins has allowed the rational design of site-specific DNA binding proteins. The ability of zinc to be bound specifically within a range of tetrahedral sites appears to be responsible for the evolution of the side range of zinc-stabilized structural domains now known to exist. The lack of redox activity for the zinc ion and its binding and exchange kinetics also may be important in the use of zinc for specific functional roles.
(2) Turovskiy Y, Chikindas ML. Zinc Lactate and Sapindin Act Synergistically with Lactocin 160 Against Gardnerella vaginalis. Probiotics Antimicrob Proteins. 2011 Jun 1;3(2):144-9. doi: 10.1007/s12602-011-9068-5
Abstract. Lactocin 160 is a vaginal probiotic-derived bacteriocin shown to selectively inhibit the growth of Gardenerella vaginalis and some other pathogens commonly associated with bacterial vaginosis. The natural origin of this peptide, its safety, and selective antimicrobial properties make it a promising candidate for successful treatment and prophylaxis of bacterial vaginosis (BV). This study evaluated interactions between lactocin 160 and four other natural antimicrobials in the ability to inhibit G. vaginalis. We report that zinc lactate and soapnut extract act synergistically with lactocin 160 against this pathogen and therefore have a potential to be successfully used as the components of the multiple-hurdle antimicrobial formulation for the treatment of BV.
(3) Suara RO, Crowe JE Jr. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Chemother. 2004 Mar;48(3):783-90.
Abstract. Zinc supplementation decreases the morbidity of lower respiratory tract infection in pediatric patients in the developing world. We sought to determine if zinc mediates a specific inhibitory effect against the major cause of pediatric lower respiratory tract disease, respiratory syncytial virus (RSV). We determined the in vitro inhibitory effect of three zinc salts (zinc acetate, lactate, and sulfate) on the replication of RSV at various concentrations of 10 and 1 mM and 100 and 10 microM. The degree of inhibition of RSV replication was examined in the presence of zinc during preincubation, adsorption, or penetration and was compared with that caused by salts of other divalent cations. Complete inhibition of RSV plaque formation was observed at 1 and 10 mM, representing reductions that were >or=10(6)-fold. At the lowest concentration tested, 10 microM, we observed >or=1000-fold reductions in RSV yield when zinc was present during preincubation, adsorption, penetration, or egress of virus. The therapeutic indices, determined as ratios of 50% toxicity concentration to 50% inhibitory concentration, were 100, 150, and 120 for zinc acetate, zinc lactate, and zinc sulfate, respectively. The inhibitory effect of zinc salts on RSV was concentration dependent and was not observed with other salts containing divalent cations such as calcium, magnesium, and manganese. RSV plaque formation was prevented by pretreatment of HEp-2 cell monolayer cultures with zinc or by addition of zinc to methylcellulose overlay media after infection. The results of this study suggest that zinc mediates antiviral activity on RSV by altering the ability of the cell to support RSV replication.
(4) McEntire JC, Montville TJ, Chikindas ML. Synergy between nisin and select lactates against Listeria monocytogenes is due to the metal cations. J Food Prot. 2003 Sep;66(9):1631-6.
Abstract. Listeria monocytogenes, a major foodborne pathogen, has been responsible for many outbreaks and recalls. Organic acids and antimicrobial peptides (bacteriocins) such as nisin are produced by lactic acid bacteria and are commercially used to control pathogens in some foods. This study examined the effects of lactic acid (LA) and its salts in combination with a commercial nisin preparation on the growth of L. monocytogenes Scott A and its nisin-resistant mutant. Because of an increase in its activity at a lower pH, nisin was more active against L. monocytogenes when used in combination with LA. Most of the salts of LA, including potassium lactate, at up to 5% partially inhibited the growth of L. monocytogenes and had no synergy with nisin. Zinc and aluminum lactate, as well as zinc and aluminum chloride (0.1%), worked synergistically with 100 IU of nisin per ml to control the growth of L. monocytogenes Scott A. No synergy was observed when zinc or aluminum lactate was used with nisin against nisin-resistant L. monocytogenes. The nisin-resistant strain was more sensitive to Zn lactate than was wild-type L. monocytogenes Scott A; however, the cellular ATP levels of the nisin-resistant strain were not significantly affected. Changes in the intracellular ATP levels of the wild-type strain support our hypothesis that pretreatment with zinc lactate sensitizes cells to nisin. The similar effects of thesalts of hydrochloric and lactic acids support the hypothesis that metal cations are responsible for synergy with nisin.
(5) Ledder RG, McBain AJ. An in vitro comparison of dentifrice formulations in three distinct oral microbiotas. Arch Oral Biol. 2012 Feb;57(2):139-47. doi: 10.1016/j.archoralbio.2011.08.004. Epub 2011 Sep 7.


![]() Zinc lactate
Zinc lactate