![]() Benzisothiazolinone
Benzisothiazolinone
Rating : 5.8
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antibacterial (1)Cons:
Allergen (1)19 pts from A_Partyns
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Benzisothiazolinone studies" about Benzisothiazolinone Review Consensus 10 by Al222 (20707 pt) | 2022-Jul-17 21:41 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Herman A, Aerts O, Jacobs MC, Scheers C, Gilissen L, Goossens A, Baeck M. Evolution of methylisothiazolinone sensitization: A Belgian multicentric study from 2014 to 2019. Contact Dermatitis. 2021 Dec;85(6):643-649. doi: 10.1111/cod.13956.
Abstract. In the 2010s an epidemic of allergic contact dermatitis to methylisothiazolinone (MI) occurred in Europe. European authorities banned the use of methylisothiazolinone in leave-on cosmetics in 2017 and limited its use in rinse-off products in 2018.
Castelli R, Scalvini L, Vacondio F, Lodola A, Anselmi M, Vezzosi S, Carmi C, Bassi M, Ferlenghi F, Rivara S, Møller IR, Rand KD, Daglian J, Wei D, Dotsey EY, Ahmed F, Jung KM, Stella N, Singh S, Mor M, Piomelli D. Benzisothiazolinone Derivatives as Potent Allosteric Monoacylglycerol Lipase Inhibitors That Functionally Mimic Sulfenylation of Regulatory Cysteines. J Med Chem. 2020 Feb 13;63(3):1261-1280. doi: 10.1021/acs.jmedchem.9b01679.
Abstract. We describe a set of benzisothiazolinone derivatives that are potent inhibitors of monoacylglycerol lipase, the primary degrading enzyme for the endocannabinoid 2-arachidonoyl-sn-glycerol (2-AG).
King N, Latheef F, Wilkinson M. Trends in preservative allergy: Benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021 Dec;85(6):637-642. doi: 10.1111/cod.13968.
Abstract. Our data show a significant increase in the prevalence of contact allergy to BIT over the last 6 years, probably as a consequence of increased use in household products.
Varga Z, Nicol E, Bouchonnet S. Photodegradation of benzisothiazolinone: Identification and biological activity of degradation products. Chemosphere. 2020 Feb;240:124862. doi: 10.1016/j.chemosphere.2019.124862.
Abstract. Compared with experimental data, the calculated oral rat LD50 values were found to be the most relevant and thus used for toxicity estimation. The photoproducts including a phenolic or a sulfino group or both functions were found potentially more toxic than benzisothiazolinone.
Kwak S, Choi YS, Na HG, Bae CH, Song SY, Kim HG, Kim YD. Benzisothiazolinone upregulates the MUC5AC expression via ERK1/2, p38, and NF-κB pathways in airway epithelial cells. Toxicol Res (Camb). 2019 Jul 16;8(5):704-710. doi: 10.1039/c9tx00135b.
Abstract. Mucus plays an important role in protecting the respiratory tract from irritants. However, mucus hypersecretion is a major indicator of airway diseases. 1,2-Benzisothiazolin-3-one, as a microbicide, induces asthmatic inflammation.
Eckhardt T, Goddard R, Lehmann C, Richter A, Sahile HA, Liu R, Tiwari R, Oliver AG, Miller MJ, Seidel RW, Imming P. Crystallographic evidence for unintended benzisothiazolinone 1-oxide formation from benzothiazinones through oxidation. Acta Crystallogr C Struct Chem. 2020 Sep 1;76(Pt 9):907-913. doi: 10.1107/S2053229620010931.
Abstract. 1,3-Benzothiazin-4-ones are a promising new class of drugs with activity against Mycobacterium tuberculosis, which have already reached clinical trials.
Garcia-Hidalgo E, Schneider D, von Goetz N, Delmaar C, Siegrist M, Hungerbühler K. Aggregate consumer exposure to isothiazolinones via household care and personal care products: Probabilistic modelling and benzisothiazolinone risk assessment. Environ Int. 2018 Sep;118:245-256. doi: 10.1016/j.envint.2018.05.047.
Abstract. A Quantitative Risk Assessment for BIT using Sensitization Assessment Factors (SAFs) indicates that around 1% of the Swiss population is at risk to be sensitized by benzisothiazolinone in cosmetics and household chemicals. For isothiazolinones in general the presented higher-tier modelling approach suggests that household cleaners are currently more important sources of exposure than cosmetics.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Benzisothiazolinone Review Consensus 19 by A_Partyns (12948 pt) | 2024-Oct-07 16:18 |
| Read the full Tiiip | (Send your comment) |
Benzisothiazolinone or Benzisothiazolin-3-one or BIT is a chemical compound and belongs to the family of isotiazolinones, heterocyclic compounds that include sulfur, nitrogen, and oxygen.
Benzisothiazolinone is a synthetic preservative widely used in cosmetic, personal care, and household cleaning products to prevent the growth of bacteria, fungi, and yeast. Due to its antimicrobial properties, it effectively protects formulations from contamination, extending the product's shelf life and ensuring safety. It is primarily used in water-based products, where the risk of microbial contamination is higher.
Chemical Composition and Structure
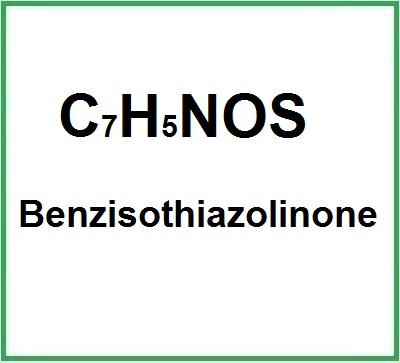
Benzisothiazolinone has the chemical formula C7H5NOS and belongs to the isothiazolinone family, known for their preservative properties. Its chemical structure includes a benzene ring attached to an isothiazolinone group, which gives it potent antibacterial and antifungal activity.
Physical Properties
Benzisothiazolinone typically appears as a white or yellowish powder but is often used in solution form in cosmetic products. It is water-soluble, making it suitable for use in aqueous formulations. It is effective at low concentrations, so only small amounts are used in products.
Production Process
Benzisothiazolinone is synthesized chemically through industrial processes that combine benzoic derivatives with sulfonating agents. The result is a stable preservative suitable for use in a wide range of formulations, including skin care, hair care, and household cleaning products.
The name 'benzisothiazolone' describes the structure of the molecule:
- "Benz-" refers to a hexagonal benzene ring of six carbon atoms, each bonded to a hydrogen atom.
- "-isothiazol-" refers to a five-membered isothiazole ring containing two carbon atoms, two nitrogen atoms and one sulphur atom.
- "-one" indicates the presence of a carbonyl group consisting of a carbon atom double bonded with an oxygen atom in the molecule.
Description of raw materials used in production:
- Benzene is a hydrocarbon compound that serves as the backbone for the benzisothiazolinone structure. It can be obtained from various sources, including crude oil or through chemical synthesis.
- Isothiazolinone. The isothiazolinone group is an essential component of benzisothiazolinone. It is typically obtained through a chemical reaction involving the condensation of primary amines with a sulfur-containing compound.
The synthesised extraction process takes place in three steps:
- Formation of the isothiazolin ring. The first step involves the reaction of an amine, such as ammonia or a primary or secondary amine, with carbon disulphide in a process known as the Willgerodt-Kindler reaction. A dithiocarbamate salt is thus formed.
- Oxidation. The dithiocarbamate salt is oxidised using hydrogen peroxide or another suitable oxidising agent. An isothiazolinone ring is thus formed.
- Aromatic substitution. The third and final step is the substitution of the benzene ring. Finally, a benzene ring is attached to the isothiazolin ring through a process known as aromatic substitution. This forms benzisothiazolinone.
It occurs in powder form with white to slightly yellow color.

(The review is constantly updated with the publication of the most recent studies.)
What it is used for and where
Benzisothiazolinone is a biocide, i.e. it can kill microorganisms. It is often used as a preservative in products such as paints, coatings and personal care products to prevent the growth of bacteria and fungi. It is effective at very low concentrations and is active against a wide range of microorganisms. However, it can cause allergic reactions in some people, so its use is regulated in many countries.
Benzisothiazolinone has antibacterial, fungicide and preservative function in household care and personal care products.
Cosmetics
Antimicrobial agent. This ingredient is able to suppress or inhibit the growth and replication of a broad spectrum of microorganisms such as bacteria, fungi and viruses by making the stratum corneum temporarily bactericidal and fungicidal.
Cosmetic safety
In its study of 26-27 June 2012 Scientific Committee on Consumer Safety determined that "The SCCS considers benzisothiazolinone safe for use as a preservative in cosmetics products up to 0.01% with respect to systemic toxicity. However, its sensitising potential is of concern." and in addition:
"Sensitisation from related isothiazolinones is an important problem in consumers. This has occurred because there has been consumer exposure before safe levels of exposure relevant to sensitisation have been established. Benzisothiazolinone is a skin sensitiser in animal models with potency similar to methylisothiazolinone.Methylisothiazolinone, at 100 ppm (0.01%) in cosmetic products is causing contact allergy and allergic contact dermatitis in the consumer. Benzisothiazolinone is known to be a sensitiser in man and has induced sensitisation at circa 20 ppm in gloves. There is no information on what may be safe levels of exposure to benzisothiazolinone in cosmetic products from the point of view of sensitisation."

Until safe levels of exposure have been established, the use of benzisothiazolinone in cosmetic products as a preservative or for other functions cannot be considered safe in relation to sensitisation.
It is used in cosmetic products such as liquid soaps, shampoos, polyvinyl chloride gloves, sunscreens etc. Typical concentrations in products are 200–400 ppm depending on the application area and the combination with other biocides.

Specific antibacterial properties
(Abstract) Gram-positive bacteria, in general, and staphylococci, in particular, are the widespread cause of nosocomial and community-acquired infections. The rapid evolvement of strains resistant to antibiotics currently in use is a serious challenge. Novel antimicrobial compounds have to be developed to fight these resistant bacteria, and sortase A, a bacterial cell wall enzyme, is a promising target for novel therapies. As a transpeptidase that covalently attaches various virulence factors to the cell surface, this enzyme plays a crucial role in the ability of bacteria to invade the host's tissues and to escape the immune response. In this study we have screened a small molecule library against recombinant Staphylococcus aureus sortase A using an in vitro FRET-based assay. The selected hits were validated by NMR methods in order to exclude false positives and to analyze the reversibility of inhibition. Further structural and functional analysis of the best hit allowed the identification of a novel class of benzisothiazolinone-based compounds as potent and promising sortase inhibitors (1).
Generic allergies
Occasionally some episode of allergy to this compound has been reported (2).
In the light of the exceptionally high rates of contact allergy to the preservative methylisothiazolinone (MI), information about cross-reactivity between MI, octylisothiazolinone (OIT) and benzisothiazolinone (BIT) this study report that the data indicate cross-reactivity between MI, OIT and BIT, when the potency of the chemical was taken into account in choice of challenge concentration. This means that MI-sensitized individuals may react to OIT and BIT if exposed to sufficient concentrations (3).
Allergy to plastic gloves (PVC)
Benzisothiazolinone is used as a slimy in the manufacture of disposable powder-free polyvinyl chloride (PVC) gloves.
Contact allergy to plastic gloves is rare. Benzisothiazolinone is a biocide that is mainly used in industrial settings. It is suspected delayed-type contact allergy to benzisothiazolinone from polyvinyl chloride (PVC) gloves in 2004. We looked through our medical records from 1991 to 2005 to find similar cases. To our knowledge, there have been no previous reports of contact allergy to antimicrobial agents in plastic gloves. Benzisothiazolinone is widely used as a biocide in the manufacture of disposable PVC gloves. Small amounts of benzisothiazolinone in the gloves may sensitize those who already have hand dermatitis (4).
Dermatitis
Occupational allergic contact dermatitis caused by benzisothiazolinone in printing ink and soap have been found (5).
The level of safety
A screening level exposure estimate was performed for several product use scenarios with sunscreen, laundry detergent, dish soap, and spray cleaner. We calculated that Benzisothiazolinone (BIT) concentrations below the following concentrations of 0.0075%, 0.035%, 0.035%, 0.021% in sunscreen, laundry detergent, dish soap, and spray cleaner, respectively, are unlikely to induce skin sensitization. We completed a pilot study consisting of bulk sample analysis of one representative product from each category labelled as containing BIT, and found BIT concentrations of 0.0009% and 0.0027% for sunscreen and dish soap, respectively. BIT was not detected in the laundry detergent and spray cleaner products above the limit of detection of 0.0006%. Based on publically available data for product formulations and our results, we were able to establish that cleaning products and sunscreens likely contain BIT at concentrations similar to or less than our calculated maximal safe concentrations and that exposures are unlikely to induce skin sensitization in most users (6).
Consumers regularly use household care and personal care products (HC&PCPs). Isothiazolinones are included in HC&PCPs as preservatives and are being held responsible for an epidemic rise in allergic contact dermatitis (ACD). The objective of this study was to assess the origin and extent of dermal exposure in order to evaluate the risk of ACD from isothiazolinones in HC&PCP. Individual-based aggregate dermal exposure to four isothiazolinones was estimated using the newly proposed Probabilistic Aggregated Consumer Exposure Model-Kinetic, Dermal (PACEM-KD) by combining the reported individual use patterns for HC&PCP in Switzerland (N = 669 (558 adults), ages 0-91) with isothiazolinone concentrations measured in products used by the individual person. PACEM-KD extends the original PACEM by considering exposure duration, product dilution and skin permeability. PACEM-KD-based higher-tier exposure on palms (99th percentile) was 15.4 ng/cm2, 1.3 ng/cm2, 0.9 ng/cm2, and 0.08 ng/cm2 for the isothiazolinones 1,2‑Benzisothiazol‑3‑(2H)‑one (BIT), 2‑Octyl‑3(2H)‑isothiazolinone (OIT), 2‑Methylisothiazolin‑3(2H)‑one (MI), and 5‑Chloro‑2‑methyl‑4‑isothiazolin‑3‑one (CMI), respectively. Major sources of exposure to BIT included all-purpose cleaners, dishwashing detergent, and kitchen cleaner, while exposure to OIT mainly stems from a fungicide. For MI, the main contributors were dishwashing detergent and all-purpose wet wipes, and for CMI all-purpose cleaner. A Quantitative Risk Assessment (QRA) for BIT using Sensitization Assessment Factors (SAFs) indicates that around 1% of the Swiss population is at risk to be sensitized by BIT in cosmetics and household chemicals. For isothiazolinones in general the presented higher-tier modelling approach suggests that household cleaners are currently more important sources of exposure than cosmetics (7).
Other studies
The regulation of 2-arachidonoylglycerol (2-AG) levels is a major issue as 2-AG has been proven to participate in numerous physiopathological phenomena such as neuroprotection or analgesia. Octhilinone, a cysteine-reagent compound, has recently been shown to inhibit in the nanomolar range monoacylglycerol lipase (MAGL), the major enzyme responsible for the degradation of 2-AG. Here, we further investigate the mechanism by which octhilinone and its benzisothiazolinone analog inhibit human MAGL. We also provide new information on the structural requirements for MAGL inhibition by these compounds. Finally, we describe for N-octylbenzisothiazolinone a mode of inhibition which is partially different from that described for octhilinone, especially with regard to the targeted cysteine residues in the vicinity of the catalytic site. (8).
Various mono- and bis-benzisothiazolone derivatives were synthesized and screened against different strains of bacteria and fungi in order to understand the effect of multiple electrophilic sulfur atoms and substitution pattern in the immediate vicinity of reactive sulfur. Preliminary studies also showed that this class of compounds have the ability to target malaria parasite with IC50 values in low micromolar range, and improvement of selectivity is possible through structure optimization (9).
Commercial applications
Preservative in Cleaning Products. Benzisothiazolinone is used in detergents, soaps, and other cleaning products to prevent the growth of microorganisms.
Preservative in Cosmetics and Skin Care Products. Used in some creams, lotions, and other skin products to extend shelf life and prevent contamination.
Preservative in Paints and Resins. Added to paints, adhesives, and resins to prevent the growth of mold and bacteria.
Preservative in Industrial Products. Used in a variety of industrial products like metalworking fluids and leather processing products.
Biocide. Benzisothiazolinone can act as an effective biocide against a variety of harmful organisms.
The most relevant studies on this chemical compound have been selected with a summary of their contents:
 |  |
 |  |
- Molecular Formula: C7H5NOS
- Molecular Weight: 151.183 g/mol
- UNII: HRA0F1A4R3
- CAS: 2634-33-5 101964-01-6 40991-37-5 54392-14-2 75037-67-1 552320-00-0 1094749-54-8 919284-21-2 1148150-72-4 1376937-61-9 1399460-92-4 934197-15-6 851309-27-8
- EC Number: 220-120-9
- PubChem Substance ID 24879952
- MDL number MFCD00127753
WHERE TO BUY (Prices are approximate and may change at any time)
Typical optimal characteristics of Benzisothiazolinone as commercial product
| Appearance | White to light yellow powder |
| Assay HPLC | ≥99.0% |
| Melting point | 154-158 °C(lit.) |
| Boiling point | 360°C |
| Density | 1.2170 |
| Flash point | 165℃ |
| Refractive index | 1.5500 |
| Solubility | Soluble in dichloromethane, dimethyl sulfoxide, methanol. |
| Chloride | 0.4%max |
| Water | ≤15 |
| Color of Alkali Solution Y | 2 |
| Storage temperature | -20°C Freezer |
Synonyms:
- 1,2-Benzisothiazol-3(2H)-one
- 1,2-Benzisothiazolin-3-one
- 1,2-benzothiazol-3-one
- benzisothiazoline-3-one
- 1,2-benzisothiazolinone
- benzisothiazolone
- Benzo[d]isothiazol-3(2H)-one
- Proxel
- 2,3-dihydro-1,2-benzothiazol-3-one
- Nipacide BIT
- Proxel AB
- Proxel XL 2
- 1,2-Benzisothiazoline-3-one
- Benzo[d]isothiazol-3-one
- Proxel PL
- Benzo[d]isothiazol-3-ol
- 3-Hydroxy-1,2-benzisothiazole
- 1,2-BENZISOTHIAZOL-3-ONE
- benzoisothiazol-3-one
- 1,2-benzoisothiazolin-3-one
- 1,2-Benzisothiazolone
- Benzisothiazolin-3-one
- Caswell No. 079A
- Caswell No. 513A
- Canguard BIT
- Proxel BD
- CCRIS 6369
- IPX
- CHEBI:167099
- Topcide 600
- Bioban BIT 20DPG
- EINECS 220-120-9
- Canguard BIT 20DPG
- EPA Pesticide Chemical Code 098901
- HRA0F1A4R3
- 1,2-Benzoisothiazoline-3-one
- 40991-37-5
- DMSMPAJRVJJAGA-UHFFFAOYSA-N
- 2,3-dihydro-3-oxo-1,2-benzisothiazole
- MLS-0254244.0001
- Benzocil
- Acticide BIT
- Denicide BIT
- Proxel CF
- Proxel TN
- Proxel XL
- Proxel BDN
- Proxel GXL
- Proxel Ultra 5
- San-aibac AP
- Proxel LV-S
- Proxel Press Paste
- Apizas AP-DS
- Acticide BW 20
- Bestcide 200K
- Nipacide BIT 20
- Parmetol B 70
- Parmetol D 11
- Proxel GXL(S)
- Proxel HL 2
- Nuosept 485
- Nuosept 491
- Nuosept 495
- XBINX
- Denicide BIT 20N
- Koralone B 119
- Nipacide BIT 10W
- Preventol BIT 20D
- Proxel BD 20
- Proxel Press Paste D
- Troysan 1050
- SD 202 (bactericide)
- Canguard Ultra BIT 20LE
- 1,2-Benzoisothiazol-3-one
- BIT 10W
- BIT 20
- 2-Thiobenzimide
- 2,3-Dihydrobenzisothiazol-3-one
- 1,2-benzisothiazole-3(2h)-one
References___________________________________________________________________
(1) Discovery and structure-activity relationship studies of irreversible benzisothiazolinone-based inhibitors against Staphylococcus aureus sortase A transpeptidase. Zhulenkovs D, Rudevica Z, Jaudzems K, Turks M, Leonchiks A. Bioorg Med Chem. 2014 Nov 1;22(21):5988-6003. doi: 10.1016/j.bmc.2014.09.011.
(2) Occupational allergic contact dermatitis caused by benzisothiazolinone in printing ink and soap. Meysman T, Goossens A.
Contact Dermatitis. 2017 Jan;76(1):51-53. doi: 10.1111/cod.12642
(3) Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Schwensen JF, Menné Bonefeld C, Zachariae C, Agerbeck C, Petersen TH, Geisler C, Bollmann UE, Bester K, Johansen JD.
Br J Dermatol. 2017 Jan;176(1):176-183. doi: 10.1111/bjd.14825.
(4) Antimicrobial allergy from polyvinyl chloride gloves. Aalto-Korte K, Alanko K, Henriks-Eckerman ML, Jolanki R. Arch Dermatol. 2006 Oct;142(10):1326-30.
1,2-benzisothiazolin-3-one in disposable polyvinyl chloride gloves for medical use. Aalto-Korte K, Ackermann L, Henriks-Eckerman ML, Välimaa J, Reinikka-Railo H, Leppänen E, Jolanki R. Contact Dermatitis. 2007 Dec;57(6):365-70.
(5) Occupational allergic contact dermatitis caused by benzisothiazolinone in printing ink and soap. Meysman T, Goossens A. Contact Dermatitis. 2017 Jan;76(1):51-53. doi: 10.1111/cod.12642
(7) Aggregate consumer exposure to isothiazolinones via household care and personal care products: Probabilistic modelling and benzisothiazolinone risk assessment. Garcia-Hidalgo E, Schneider D, von Goetz N, Delmaar C, Siegrist M, Hungerbühler K. Environ Int. 2018 Sep;118:245-256. doi: 10.1016/j.envint.2018.05.047.
(8) Benzisothiazolinone as a useful template for the design of new monoacylglycerol lipase inhibitors: investigation of the target residues and comparison with octhilinone. Matuszak N, Es Saadi B, Labar G, Marchand-Brynaert J, Lambert DM. Bioorg Med Chem Lett. 2011 Dec 15;21(24):7321-4. doi: 10.1016/j.bmcl.2011.10.026.
(9) Broad spectrum anti-infective properties of benzisothiazolones and the parallels in their anti-bacterial and anti-fungal effects. Gopinath P, Yadav RK, Shukla PK, Srivastava K, Puri SK, Muraleedharan KM. Bioorg Med Chem Lett. 2017 Mar 1;27(5):1291-1295. doi: 10.1016/j.bmcl.2017.01.027.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances:
Last update: 2023-08-22 11:58:34 | Chemical Risk: |