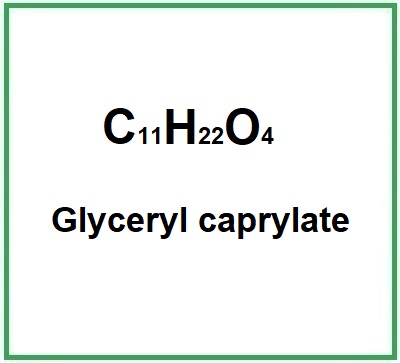
![]() Glyceryl caprylate
Glyceryl caprylate
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antimicrobial (1)7 pts from CarPas
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Glyceryl caprylate Review Consensus 7 by CarPas (5242 pt) | 2024-Sep-27 17:57 |
| Read the full Tiiip | (Send your comment) |
Glyceryl Caprylate is an ester derived from glycerin and caprylic acid, a medium-chain fatty acid. This ingredient is widely recognized for its multifunctional properties, serving as an emollient, emulsifier, and deodorant in cosmetic formulations. Glyceryl Caprylate helps to improve the texture and stability of products while providing moisturizing benefits. It is effective in preventing moisture loss and enhancing the overall skin feel, making it a valuable addition to various personal care products.
Chemical Composition and Structure
Glyceryl Caprylate consists of:
- Glycerin: A humectant that attracts moisture and helps maintain skin hydration.
- Caprylic Acid: A fatty acid known for its emollient properties and ability to improve skin texture.
- Ester Linkage: The combination of glycerin and caprylic acid through an ester bond contributes to its functional properties.
This unique composition allows Glyceryl Caprylate to provide both moisturizing and stabilizing effects in formulations.
Physical Properties
Appearance: Typically a clear to pale yellow liquid or viscous substance.
Solubility: Soluble in oils and fats; limited solubility in water.
pH: Generally neutral, making it compatible with a wide range of cosmetic formulations.
Odor: Mild, often with a slight fatty scent.
Stability: Stable under normal storage conditions; should be protected from excessive heat and moisture.
Production Process
Synthesis: Glyceryl Caprylate is produced through the esterification of glycerin and caprylic acid, typically using heat and catalysts to facilitate the reaction.
Purification: The resulting product is purified to remove any unreacted materials and by-products, ensuring a high-quality ingredient.
Formulation: Purified Glyceryl Caprylate is then incorporated into various cosmetic products to enhance their emollient and emulsifying properties.
Applications
Medical: Occasionally used in topical formulations for its soothing properties and ability to prevent skin irritation.
Cosmetics: Commonly found in lotions, creams, hair conditioners, and deodorants for its moisturizing, emulsifying, and stabilizing benefits. It enhances the texture and sensory experience of products.
INCI Functions:
Deodorant agent. When substances that give off an unpleasant odour are included in cosmetic formulations (typical examples are methyl mercaptan and hydrogen sulphide derived from garlic), deodorants attenuate or eliminate the unpleasant exhalation. It helps counteract the formation of bad odours on body surfaces.
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Industrial Uses: May be employed in formulations requiring effective emulsification and stabilization properties.
Environmental and Safety Considerations
Glyceryl Caprylate is generally regarded as safe for use in cosmetics when applied according to recommended guidelines. It is well-tolerated by most skin types, including sensitive skin. Responsible sourcing and formulation practices are essential to ensure that the ingredient is free from harmful contaminants and produced sustainably.
 |  |
Molecular Formula C11H22O4
Molecular Weight 218.293
CAS 26402-26-6
EINECS 247-668-1
- 2,3-Dihydroxypropyl octanoate
- glyceryl-1-monooctanoate
- glycerol-1-monooctanoate
- glyceryl monocaprylate
- mono-octanoin
- monocaprylin
- monoctanoin
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Is Glyceryl caprylate safe ?" about Glyceryl caprylate Review Consensus 8 by CarPas (5242 pt) | 2024-Sep-27 17:53 |
| Read the full Tiiip | (Send your comment) |
Glyceryl caprylate is a glycerol monoester.
The family of glycerol monoesters includes 43 products, 20 of which are commonly used in cosmetics :
Glyceryl Laurate, Glyceryl Alginate, Glyceryl Arachidonate, Glyceryl Behenate, Glyceryl Caprylate, Glyceryl Caprylate/Caprate, Glyceryl Cocoate, Glyceryl Erucate, Glyceryl Hydroxystearate, Glyceryl Isostearate, Glyceryl Lanolate, Glyceryl Linoleate, Glyceryl Linolenate, Glyceryl Myristate, Glyceryl Oleate/Elaidate, Glyceryl Palmitate, Glyceryl Polyacrylate, Glyceryl Rosinate, Glyceryl Stearate/Acetate, and Glyceryl Undecylenate. Normally, the concentration of these components is 12% in cosmetic products. The function of these components is emollient, surfactant and emulsifier.The Glyceryl momnoesters are not pure, but because they are metabolized into free fatty acids and glycerol, they are mainly mixtures of mono-, di- and tri-esters (1).
The Glyceryl caprylate does not seem to have, so far, low or acute toxicity.
This study describes a new alternative combination preservative containing caprylyl glycol, phenethyl alcohol and glyceryl caprylate and investigates the effects of the particle size of the emulsion droplet on the anti-microbial activity of the said blend in the formulation. The synergistic performance of caprylyl glycol, phenethyl alcohol and glyceryl caprylate and the anti-microbial activity of their blend suggest that their combination is effective and exhibits broad-spectrum anti-microbial activity. Furthermore, the results show a positive correlation between the anti-microbial activity of the preservative and the particle size of the emulsion droplets in the range of 100-900 nm, when the same concentration of the blend is used in the same formulation. The particle size of the emulsion droplets is demonstrated to be a newly discovered factor that influences the preservation of cosmetic products (2).
Molecular Formula: C11H22O4
Molecular Weight: 218.293
CAS : 26402-26-6
Synonyms :
- Monocaprylyn, Glycerylcaprylate, Monooctanoin,
- Monoesterwith1,2,3-propanetriol
References____________________________________________________________________
(1) Final report of the amended safety assessment of Glyceryl Laurate, Glyceryl Laurate SE, Glyceryl Laurate/Oleate, Glyceryl Adipate, Glyceryl Alginate, Glyceryl Arachidate, Glyceryl Arachidonate, Glyceryl Behenate, Glyceryl Caprate, Glyceryl Caprylate, Glyceryl Caprylate/Caprate, Glyceryl Citrate/Lactate/Linoleate/Oleate, Glyceryl Cocoate, Glyceryl Collagenate, Glyceryl Erucate, Glyceryl Hydrogenated Rosinate, Glyceryl Hydrogenated Soyate, Glyceryl Hydroxystearate, Glyceryl Isopalmitate, Glyceryl Isostearate, Glyceryl Isostearate/Myristate, Glyceryl Isostearates, Glyceryl Lanolate, Glyceryl Linoleate, Glyceryl Linolenate, Glyceryl Montanate, Glyceryl Myristate, Glyceryl Isotridecanoate/Stearate/Adipate, Glyceryl Oleate SE, Glyceryl Oleate/Elaidate, Glyceryl Palmitate, Glyceryl Palmitate/Stearate, Glyceryl Palmitoleate, Glyceryl Pentadecanoate, Glyceryl Polyacrylate, Glyceryl Rosinate, Glyceryl Sesquioleate, Glyceryl/Sorbitol Oleate/Hydroxystearate, Glyceryl Stearate/Acetate, Glyceryl Stearate/Maleate, Glyceryl Tallowate, Glyceryl Thiopropionate, and Glyceryl Undecylenate.
Int J Toxicol. 2004;23 Suppl 2:55-94.
Abstract. The safety of 43 glyceryl monoesters listed as cosmetic ingredients was reviewed in a safety assessment completed in 2000. Additional safety test data pertaining to Glyceryl Rosinate and Glyceryl Hydrogenated Rosinate were received and served as the basis for this amended report. Glyceryl monoesters are used mostly as skin-conditioning agents--emollients and/or surfactant--emulsifying agents in cosmetics. The following 20 glyceryl monoesters are currently reported to be used in cosmetics: Glyceryl Laurate, Glyceryl Alginate, Glyceryl Arachidonate, Glyceryl Behenate, Glyceryl Caprylate, Glyceryl Caprylate/Caprate, Glyceryl Cocoate, Glyceryl Erucate, Glyceryl Hydroxystearate, Glyceryl Isostearate, Glyceryl Lanolate, Glyceryl Linoleate, Glyceryl Linolenate, Glyceryl Myristate, Glyceryl Oleate/Elaidate, Glyceryl Palmitate, Glyceryl Polyacrylate, Glyceryl Rosinate, Glyceryl Stearate/Acetate, and Glyceryl Undecylenate. Concentration of use data received from the cosmetics industry in 1999 indicate that Glyceryl Monoesters are used at concentrations up to 12% in cosmetic products. Glyceryl Monoesters are not pure monoesters, but are mostly mixtures with mono-, di-, and tri-esters. The purity of commercial and conventional Monoglyceride (Glyceryl Monoester) is a minimum of 90%. Glyceryl Monoesters (monoglycerides) are metabolized to free fatty acids and glycerol, both of which are available for the resynthesis of triglycerides. Glyceryl Laurate enhanced the penetration of drugs through cadaverous skin and hairless rat skin in vitro and has been described as having a wide spectrum of antimicrobial activity. A low-grade irritant response was observed following inhalation of an aerosol containing 10% Glyceryl Laurate by test animals. Glyceryl monoesters have little acute or short-term toxicity in animals, and no toxicity was noted following chronic administration of a mixture consisting mostly of glyceryl di- and mono- esters. Glyceryl Laurate did have strong hemolytic activity in an in vitro assay using sheep erythrocytes. Glyceryl Laurate, Glyceryl Isostearate, or Glyceryl Citrate/Lactate/Linoleate/Oleate were not classified as ocular irritants in rabbits. Undiluted glyceryl monoesters may produce minor skin irritation, especially in abraded skin, but in general these ingredients are not irritating at concentrations used in cosmetics. Glyceryl monoesters are not sensitizers, except that Glyceryl Rosinate and Hydrogenated Glyceryl Rosinate may contain residual rosin, which can cause allergic reactions. These ingredients are not photosensitizers. Glyceryl Citrate/Lactate/Linoleate/Oleate was not mutagenic in the Ames test system. Glyceryl Laurate exhibited antitumor activity and Glyceryl Stearate was negative in a tumor promotion assay. At concentrations higher than used in cosmetics, Glyceryl Laurate did cause moderate erythema in human repeat-insult patch test (RIPT) studies, but the other glyceryl monoesters tested failed to produce any significant positive reactions. Glyceryl Rosinate was irritating to animal skin at 50%, but did not produce sensitization in clinical tests at concentrations up to 10% and covered with semioccluded patches. There is reported use of Glyceryl Rosinate at 12%in mascara, which is somewhat higher than the concentration in the clinical testing. It was reasoned that the available data do support the safety of this use because there would be minimal contact with the skin and no occlusion. The safety of Arachidonic Acid was not documented and substantiated for cosmetic product use in an earlier safety assessment and those same safety questions apply to Glyceryl Arachidonate. Based on these data, the Cosmetic Ingredient Review (CIR) Expert Panel found that these glyceryl monoesters are safe as cosmetic ingredients in the present practices of use and concentration: except that the available data are insufficient to support the safety of Glyceryl Arachidonate. Additional data needed to support the safety of Glyceryl Arachidonate include (1) dermal absorption data; and, based on the results of the absorption studies, there may be a need for (2) immunomodulatory data; (3) carcinogenicity and photocarcinogenicity data; and (4) human irritation, sensitization, and photosensitization data.
(2) Fang B, Yu M, Zhang W, Wang F. A new alternative to cosmetics preservation and the effect of the particle size of the emulsion droplets on preservation efficacy. Int J Cosmet Sci. 2016 Oct;38(5):496-503. doi: 10.1111/ics.12317.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2018-10-30 19:16:13 | Chemical Risk: |