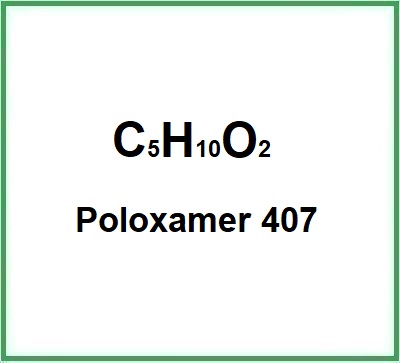
![]() Poloxamer 407
Poloxamer 407
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
9 pts from A_Partyns
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Poloxamer 407 studies" about Poloxamer 407 Review Consensus 10 by Ark90 (12432 pt) | 2023-Jun-23 21:31 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Giuliano E, Paolino D, Fresta M, Cosco D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines (Basel). 2018 Dec 29;6(1):7. doi: 10.3390/medicines6010007.
Abstract. Hydrogels are three-dimensional networks of hydrophilic polymers able to absorb and retain a considerable amount of water or biological fluid while maintaining their structure. Among these, thermo-sensitive hydrogels, characterized by a temperature-dependent sol⁻gel transition, have been massively used as drug delivery systems for the controlled release of various bioactives. Poloxamer 407 (P407) is an ABA-type triblock copolymer with a center block of hydrophobic polypropylene oxide (PPO) between two hydrophilic polyethyleneoxide (PEO) lateral chains. Due to its unique thermo-reversible gelation properties, P407 has been widely investigated as a temperature-responsive material. The gelation phenomenon of P407 aqueous solutions is reversible and characterized by a sol⁻gel transition temperature. The nanoencapsulation of drugs within biocompatible delivery systems dispersed in P407 hydrogels is a strategy used to increase the local residence time of various bioactives at the injection site. In this mini-review, the state of the art of the most important mixed systems made up of colloidal carriers localized within a P407 hydrogel will be provided in order to illustrate the possibility of obtaining a controlled release of the entrapped drugs and an increase in their therapeutic efficacy as a function of the biomaterial used.
Giuliano E, Paolino D, Fresta M, Cosco D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics. 2018 Sep 12;10(3):159. doi: 10.3390/pharmaceutics10030159.
Abstract. Poloxamer 407, also known by the trademark Pluronic® F127, is a water-soluble, non-ionic triblock copolymer that is made up of a hydrophobic residue of polyoxypropylene (POP) between the two hydrophilic units of polyoxyethylene (POE). Poloxamer 407-based hydrogels exhibit an interesting reversible thermal characteristic. That is, they are liquid at room temperature, but they assume a gel form when administered at body temperature, which makes them attractive candidates as pharmaceutical drug carriers. These systems have been widely investigated in the development of mucoadhesive formulations because they do not irritate the mucosal membranes. Based on these mucoadhesive properties, a simple administration into a specific compartment should maintain the required drug concentration in situ for a prolonged period of time, decreasing the necessary dosages and side effects. Their main limitations are their modest mechanical strength and, notwithstanding their bioadhesive properties, their tendency to succumb to rapid elimination in physiological media. Various technological approaches have been investigated in the attempt to modulate these properties. This review focuses on the application of poloxamer 407-based hydrogels for mucosal drug delivery with particular attention being paid to the latest published works.
Chen Y, Lee JH, Meng M, Cui N, Dai CY, Jia Q, Lee ES, Jiang HB. An Overview on Thermosensitive Oral Gel Based on Poloxamer 407. Materials (Basel). 2021 Aug 12;14(16):4522. doi: 10.3390/ma14164522.
Abstract. In this review, we describe the application of thermosensitive hydrogels composed of poloxamer in medicine, especially for oral cavities. Thermosensitive hydrogels remain fluid at room temperature; at body temperature, they become more viscous gels. In this manner, the gelling system can remain localized for considerable durations and control and prolong drug release. The chemical structure of the poloxamer triblock copolymer leads to an amphiphilic aqueous solution and an active surface. Moreover, the poloxamer can gel by forming micelles in an aqueous solution, depending on its critical micelle concentration and critical micelle temperature. Owing to its controlled-release effect, a thermosensitive gel based on poloxamer 407 (P407) is used to deliver drugs with different characteristics. As demonstrated in studies on poloxamer formulations, an increase in gelling viscosity decreases the drug release rate and gel dissolution time to the extent that it prolongs the drug's duration of action in disease treatment. This property is used for drug delivery and different therapeutic applications. Its unique route of administration, for many oral diseases, is advantageous over traditional routes of administration, such as direct application and systemic treatment. In conclusion, thermosensitive gels based on poloxamers are suitable and have great potential for oral disease treatment.
Tundisi LL, Yang R, Borelli LPP, Alves T, Mehta M, Chaud MV, Mazzola PG, Kohane DS. Enhancement of the Mechanical and Drug-Releasing Properties of Poloxamer 407 Hydrogels with Casein. Pharm Res. 2021 Mar;38(3):515-522. doi: 10.1007/s11095-021-03017-9.
Abstract. Purpose: Topical therapy of local disease (e.g. skin) is advantageous over oral therapy since there is less systemic drug distribution (so fewer side-effects), no first-pass effect, etc. However, patient compliance with topical therapy can be poor as it may require many applications a day and can last months. Here we propose a topical controlled release formulation with thermoresponsive gelation at body temperature and improved adhesiveness, making it easier to remain in contact with the body. Methods: The formulation contains two excipients, poloxamer 407 (P407) and casein. Casein can modify the properties of the hydrogel through molecular entanglement. In addition, tissue reaction and drug release profile were evaluated. Results: Changes in casein concentration affected adhesive strength, viscosity, mechanical properties and drug release, presumably by hydrophobic interactions between casein and P407. Two different concentrations of P407 were tested with two different concentrations of casein. Formulations containing 5% and 10% casein released 80% of model drug in 48 h, while formulations without casein released the same fraction in around 24 h hours. Formulations with 10% casein had almost twice the adhesive strength of those without casein. Conclusions: Addition of casein modified the mechanical properties and drug release rate of the hydrogel. There was no inflammation or injury after brief exposure in vivo.
Chen Z, Higashi K, Ueda K, Moribe K. Transition from Amorphous Cyclosporin A Nanoparticles to Size-Reduced Stable Nanocrystals in a Poloxamer 407 Solution. Mol Pharm. 2021 Nov 29. doi: 10.1021/acs.molpharmaceut.1c00721.
Abstract. Amorphous drug nanoparticles usually exhibit low storage stability. A comprehensive understanding of the molecular states and physicochemical properties of the product is indispensable for designing stable formulations. In the present study, an amorphous cyclosporin A (CyA) nanosuspension with a mean particle size of approximately 370 nm was prepared by wet bead milling with poloxamer 407 (P407). Interestingly, the prepared amorphous CyA nanoparticles were transformed into uniform CyA nanocrystals with a reduced mean particle size of approximately 200 nm during storage at 25 °C. The CyA nanocrystals were stably maintained for at least 1 month. The particle morphologies and molecular structures of the CyA nanosuspensions before and after storage were thoroughly characterized by cryogenic transmission electron microscopy and magic-angle spinning nuclear magnetic resonance spectroscopy, respectively. They revealed that the freshly prepared amorphous CyA nanoparticles (∼370 nm) were secondary particles composed of aggregated primary particles with an estimated size of 50 nm. A portion of P407 was found to be entrapped at the gaps between the primary particles due to aggregation, while most of P407 was dissolved in the solution either adsorbing at the solid/liquid interface or forming polymeric micelles. The entrapped P407 is considered to play an important role in the destabilization of the amorphous CyA nanoparticles. The resultant CyA nanocrystals (∼200 nm) were uniform single crystals of Form 2 hydrate and showed corner-truncated bipyramidal features. Owing to the narrow particle size distribution of the CyA nanocrystals, the rate of Ostwald ripening was slow, giving long-term stability to the CyA nanocrystals. This study provides new insights into the destabilization mechanism of amorphous drug nanoparticles.
Fakhari A, Corcoran M, Schwarz A. Thermogelling properties of purified poloxamer 407. Heliyon. 2017 Aug 30;3(8):e00390. doi: 10.1016/j.heliyon.2017.e00390.
Abstract. Poloxamers are triblock copolymers with a center block of hydrophobic polypropylene oxide (PPO) flanked by two hydrophilic polyethyleneoxide (PEO) blocks. Among this family of copolymers, poloxamer 407 is a non-ionic surfactant with reversible gelation properties above a particular polymer concentration and a particular temperature. Easy preparation of poloxamer 407 based sterile injectable formulations have made this copolymer a good candidate for drug delivery, specifically when controlled release of the drug is required. Previously, the applications of compendial poloxamer 407 preparations were demonstrated; however, low viscosity, poor elasticity, and sol-to-gel transition temperature (Tsol-gel) over a wide temperature range were observed. A purification process was introduced to eliminate impurities and low molecular weight copolymer molecules from the compendial poloxamer 407 resulting in higher viscosity values with Tsol-gel in a narrow temperature range. Here, poloxamer 407 was purified based on the proposed process and the rheological and analytical evaluation of the purified poloxamer 407 was conducted and compared to unpurified, compendial poloxamer 407. Then, the impact of poloxamer 407 concentration on gel formation was evaluated. For drug delivery applications, the effect of relevant buffer salts and the effect of addition of ethanol to the poloxamer 407 solutions were rheologically evaluated.
Ullah KH, Raza F, Munawar SM, Sohail M, Zafar H, Zafar MI, Ur-Rehman T. Poloxamer 407 Based Gel Formulations for Transungual Delivery of Hydrophobic Drugs: Selection and Optimization of Potential Additives. Polymers (Basel). 2021 Sep 30;13(19):3376. doi: 10.3390/polym13193376.
Abstract. The current study aimed to develop poloxamer 407 (P407) gel for transungual delivery of antifungal hydrophobic drugs with sufficient gel strength and drug loading. Gel strength and drug loading of P407 gel was improved by use of functional additives. Hydration enhancement effect was used to select optimum nail penetration enhancer. Face-centered central composite design (FCCCD) was used to observe the effect of the selected penetration enhancer (thioglycolic acid (TGA)) and cosolvent (ethanol) on gelation behavior to develop formulation with enough loading of hydrophobic drug, i.e., terbinafine HCl (TBN), and its permeation across the nail plate without compromising on gel strength. It was observed that increasing concentration of P407 and TGA significantly reduced gelation temperature and enhanced the gel strength of P407 gel and can be used to improve P407 gel strength. Under the scanning electron microscope, the significant effect of TGA as an ungual penetration enhancer was observed on the morphology of the nail plate. Optimized P407 gel prepared with modified cold method showed a gelation temperature of 8.7 ± 0.16 °C, gel strength of 122 ± 7.5 s and drug loading of 1.2% w/w, which was four times more than the drug loading in the gels prepared with conventional cold method. Rheological behavior was pseudoplastic with 47.75 ± 3.48% of gel erosion after 12 washings and 67.21 ± 2.16% of drug release after 12 h. A cumulative amount of TBN permeated from P407 gel with and without PE after 24 h was 27.30 ± 4.18 and 16.69 ± 2.31 µg/cm2, respectively. Thioglycolic acid can be used as a nail penetration enhancer without the chemical modification or addition of extra additives while retaining the gel strength. Water miscible cosolvents with moderate evaporability such as ethanol, can be incorporated to P407 gel by minor modification in method of preparation to load the required dose of hydrophobic drugs. Developed P407 gel formulation with sufficient gel strength and drug loading will be a promising carrier for transungual delivery of hydrophobic antifungal agents.
Zambanini T, Borges R, de Souza ACS, Justo GZ, Machado J Jr, de Araujo DR, Marchi J. Holmium-Containing Bioactive Glasses Dispersed in Poloxamer 407 Hydrogel as a Theragenerative Composite for Bone Cancer Treatment. Materials (Basel). 2021 Mar 17;14(6):1459. doi: 10.3390/ma14061459.
Abstract. Holmium-containing bioactive glasses can be applied in bone cancer treatment because the holmium content can be neutron activated, having suitable properties for brachytherapy applications, while the bioactive glass matrix can regenerate the bone alterations induced by the tumor. To facilitate the application of these glasses in clinical practice, we proposed a composite based on Poloxamer 407 thermoresponsive hydrogel, with suitable properties for applications as injectable systems. Therefore, in this work, we evaluated the influence of holmium-containing glass particles on the properties of Poloxamer 407 hydrogel (20 w/w.%), including self-assembly ability and biological properties. 58S bioactive glasses (58SiO2-33CaO-9P2O5) containing different Ho2O3 amounts (1.25, 2.5, 3.75, and 5 wt.%) were incorporated into the hydrogel. The formulations were characterized by scanning electron microscopy, differential scanning calorimetry, rheological tests, and [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] MTT cell viability against pre-osteoblastic and osteosarcoma cells. The results evidenced that neither the glass particles dispersed in the hydrogel nor the holmium content in the glasses significantly influenced the hydrogel self-assembly ability (Tmic ~13.8 °C and Tgel ~20 °C). Although, the glass particles considerably diminished the hydrogel viscosity in one order of magnitude at body temperature (37 °C). The cytotoxicity results evidenced that the formulations selectively favored pre-osteoblastic cell proliferation and osteosarcoma cell death. In conclusion, the formulation containing glass with the highest fraction of holmium content (5 wt.%) had the best biological results outcomes aiming its application as theragenerative materials for bone cancer treatment.
Vanderstraeten MCM, Gutermuth J, Grosber M. Contact anaphylaxis to poloxamer 188 and 407 in a periodontal gel. Contact Dermatitis. 2021 Mar 16. doi: 10.1111/cod.13834.
Borges R, Kai KC, Lima CA, Zezell DM, de Araujo DR, Marchi J. Bioactive glass/poloxamer 407 hydrogel composite as a drug delivery system: The interplay between glass dissolution and drug release kinetics. Colloids Surf B Biointerfaces. 2021 Oct;206:111934. doi: 10.1016/j.colsurfb.2021.111934.
Bassi da Silva J, da Silva Souza Campanholi K, Braga G, de Souza PR, Caetano W, Cook MT, Bruschi ML. The effect of erythrosine-B on the structuration of poloxamer 407 and cellulose derivative blends: In silico modelling supporting experimental studies. Mater Sci Eng C Mater Biol Appl. 2021 Nov;130:112440. doi: 10.1016/j.msec.2021.112440.
Moura S, Noro J, Cerqueira P, Silva C, Cavaco-Paulo A, Loureiro A. Poloxamer 407 based-nanoparticles for controlled release of methotrexate. Int J Pharm. 2020 Feb 15;575:118924. doi: 10.1016/j.ijpharm.2019.118924.
Minnelli C, Moretti P, Fulgenzi G, Mariani P, Laudadio E, Armeni T, Galeazzi R, Mobbili G. A Poloxamer-407 modified liposome encapsulating epigallocatechin-3-gallate in the presence of magnesium: Characterization and protective effect against oxidative damage. Int J Pharm. 2018 Dec 1;552(1-2):225-234. doi: 10.1016/j.ijpharm.2018.10.004.
Bobbala S, Gibson B, Gamble AB, McDowell A, Hook S. Poloxamer 407-chitosan grafted thermoresponsive hydrogels achieve synchronous and sustained release of antigen and adjuvant from single-shot vaccines. Immunol Cell Biol. 2018 Jul;96(6):656-665. doi: 10.1111/imcb.12031.
Choi JE, Lee JH, Chang SY, Lee MY, Jung JY. Clinical Implications of Poloxamer 407 as Packing Material in an Animal Model. Audiol Neurootol. 2019;24(2):100-108. doi: 10.1159/000500661.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Poloxamer 407 Review Consensus 9 by A_Partyns (12948 pt) | 2024-Oct-08 16:35 |
| Read the full Tiiip | (Send your comment) |
Poloxamer 407, commercially known as Pluronic™ F127, is a chemical compound, surfactant, a nonionic triblock copolymer widely used as an emulsifier, solubilizer, and surfactant in various industries, including cosmetics, pharmaceuticals, and personal care products. It belongs to the poloxamer family, which consists of block copolymers made from polyoxyethylene and polyoxypropylene. Poloxamer 407 is known for its ability to form micelles in aqueous solutions and act as a stabilizer in emulsions and gels.
Chemical Composition and Structure
Poloxamer 407 is composed of a central hydrophobic block of polyoxypropylene (PPO) sandwiched between two hydrophilic blocks of polyoxyethylene (PEO). The chemical structure is represented as PEO-PPO-PEO, which allows the polymer to act both as a solubilizing agent for hydrophobic substances and as an emulsifier in formulations where oil and water need to mix. The numbers "98;67" indicate the relative molecular weights of the polyoxyethylene and polyoxypropylene blocks.
Physical Properties
Poloxamer 407 typically appears as a white or off-white powder or granules, which are highly soluble in water. When mixed with water, it forms clear, thermoreversible gels, which means it is liquid at cooler temperatures and becomes more gel-like at higher temperatures. This property makes it ideal for use in formulations requiring temperature-sensitive viscosities. Poloxamer 407 is also known for its non-toxic and non-irritating characteristics, making it suitable for use in various skin and hair care products.
Production Process
Poloxamer 407 is synthesized through the sequential polymerization of ethylene oxide and propylene oxide. This process creates alternating hydrophilic and hydrophobic segments, resulting in a block copolymer with surfactant properties. The polymer is purified to remove residual monomers and other impurities, ensuring its safety and effectiveness in consumer products.
The name describes the structure of the molecule:
- Poloxamer is a type of non-ionic triblock copolymer consisting of a hydrophobic central polyoxypropylene (propylene oxide) chain flanked by two hydrophilic polyoxyethylene (ethylene oxide) chains.
- 407 refers to the specific type of poloxamer, which is defined by the length of the polyoxypropylene and polyoxyethylene chains. In the case of poloxamer 407, the central polyoxypropylene chain consists of approximately 56 propylene oxide units and each polyoxyethylene chain consists of approximately 101 ethylene oxide units.
The raw materials for the production of Poloxamer 407 are:
- Propylene oxide - This is a chemical compound used to form the central hydrophobic chain of polyoxypropylene. Propylene oxide is a colorless volatile liquid.
- Ethylene oxide - This is a chemical compound used to form the hydrophilic chains of polyoxyethylene.
It is obtained by polymerisation of ethylene oxide and propylene oxide in liquid form at a controlled temperature with a catalyst such as potassium hydroxide or sodium hydroxide. The synthesis process consists of the following steps:
It is obtained by polymerisation of ethylene oxide and propylene oxide in liquid form at a controlled temperature with a catalyst such as potassium hydroxide or sodium hydroxide. The synthesis process consists of the following steps:
- Reaction. Propylene oxide is reacted with a suitable initiator, such as propylene glycol, in the presence of a catalyst to form the polyoxypropylene core. The reaction takes place at high temperature and under pressure.
- Polymerisation. Ethylene oxide is added to the reaction and attaches to the ends of the polyoxypropylene core, forming polyoxyethylene building blocks. The reaction is catalysed and takes place under pressure and at high temperature. Polymerisation is stopped once the desired molecular weights for the polyoxypropylene and polyoxyethylene sections are reached.
- Purification. The resulting poloxamer is purified by a precipitation and filtration process. Ethylene oxide impurities should have been removed.
It comes in the form of fine white powder.

What it is used for and where
Cosmetics
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Cosmetic safety. In cosmetics, Poloxamer 407 is considered safe, however, as with any cosmetic ingredient, some people may be sensitive or have individual allergic reactions, so it is always advisable to test products on the skin before particularly intensive use.
Food
Among a number of natural and synthetic polymers used to form polymeric micelles, Poloxamer 407, a US Food and Drug Administration-approved polymer, is the most attractive due to its biocompatibility, biodegradability, and low toxicity (1).
Poloxamer 407 is used as an emulsifier and stabiliser in creams, sauces, ice cream, fruit drinks where it improves appearance, stability and consistency, promotes solubilisation of lipophilic compounds, improves distribution and bioavailability.
Medical
On polyoxamers, scientific research has identified a potential use, thanks to their low toxicity, solubilising capacity, compatibility with various excipients and biomolecules, as a biomaterial to obtain hydrogels for drug release and in particular Poloxamer 407 is used as a carrier, thanks to its amphiphilic nature, to improve the solubility of poorly water-soluble drugs with particular warning on the solubilisation of active ingredients.
It is used, due to its viscosity and ability to form a protective film on the ocular surface as an ocular lubricant in eye drops that help alleviate dry eye symptoms, improve comfort and as an artificial tear.
Poloxamer 407 is a cleaning agent that can remove plaque and debris from ocular surfaces, contact lenses or medical devices.
It helps protect cells during the freezing and thawing process by acting as a cellular cryopreservative, reducing cell damage and preserving cell viability.
It also has a function as a stabiliser for emulsions, suspensions and colloidal systems improving the stability of pharmaceutical formulations and preventing phase separation and a gelling function with the ability to form reversible gels at room temperature useful in ophthalmology.
It is a non-ionic surfactant that facilitates the emulsification and homogeneous distribution of ingredients in pharmaceutical formulations.
The safety and efficacy of this polymer as a temporary embolic agent were analysed and evaluated positively in this study on temporary vascular occlusion (1).
Poloxamer 407 shows some thermoreversible properties of extreme interest in optimising drug formulation (3).
Poloxamer 407 and vitamin E TPG (D-α-tocopheryl polyethylene glycol succinate) are polymers widely used as drug carriers and excipients to improve drug retention and stability times (4) and oral delivery of antibiofilm peptides (5).
An interesting potential application of Poloxamer 407 concerns 'phacoemulsification' (ultrasound) to protect the corneal endothelium (6).
Safety
Available data show that Poloxamers introduced into the body through different routes than dermal exposure have a rapid clearance from the body, suggesting that they are safe as used (7).

Allergic Reactions
Allergic reactions to Poloxamer 407 are rare due to its non-irritating nature. However, individuals with highly sensitive skin or known allergies to specific chemicals should perform patch tests before using products containing Poloxamer 407.
Toxicity and Carcinogenicity
There is no evidence that Poloxamer 407 is toxic or carcinogenic. It has been thoroughly studied for its use in pharmaceuticals and cosmetics, and it is considered safe when used in appropriate concentrations. However, at very high doses, it has been observed to potentially cause hyperlipidemia in animal studies, though this effect is not typically seen in standard cosmetic or pharmaceutical uses.
Environmental and Safety Considerations
Poloxamer 407 is biodegradable and does not pose significant environmental risks. It breaks down into non-toxic components, but, like most surfactants, care should be taken to dispose of products containing it responsibly to prevent water contamination.
Regulatory Status
Poloxamer 407 is approved for use in cosmetics, pharmaceuticals, and personal care products by regulatory bodies such as the European Union and the Food and Drug Administration (FDA) in the United States. It is listed as a safe ingredient in concentrations typically used in these products.
The most relevant studies on this chemical compound have been selected with a summary of their contents:

Optimal typical characteristics of Poloxamer 407 commercial product
| Clarity and color of solution | Clear and colorless |
| Acid | Red solution |
| Average molecular weight | 9480 g/mol-14500 g/mol |
| Ph value (25g/l in water) | 5.0-7.0 |
| Ph value (100g/l in water) | 5.0-7.0 |
| Oxyethylene | 71.5%-74.9% |
| Total ash | <0.4% |
| Residue on ignition | <0.30 |
| Insaturation | 0.031mEq/G-0.65mEq/ |
| Water | <0.75% |
| Heavy metals | <0.002% |
| BHT stabilizer | 50ppm-25ppm |
| Congealing point | 50°C-62°C |
| 1.4 dioxane | <5.0ppm |
| Ethylene oxide | <1.0ppm |
| Propylene oxide | <5.0ppm |
| Arsenic | <2ppm |
Synonyms:
Adeka Pluronic F 108 Antarox Cirrasol ALN-WS Detalan Epan 485 Epan 710 Epan 785 Ethylene oxide-propylene oxide block copolymer dipropylene glycol ether Ethylene oxide-propylene oxide block copolymer ether with ethylene glycol F-108 Lutrol F Pluracare L 61 Poloxalene Poloxalkol Poloxamene Poloxamer 188 Poly(ethylene oxide-co-propylene oxide) Polyoxamer Polyoxyethylene - polyoxypropylene block copolymer Polyoxyethylene - polyoxypropylene copolymer Tergitol XH Therabloat
- Molecular Formula: C5H10O2
- Molecular Weight: 102,133
- CAS: 9003-11-6
- UNII
- EC Number: 923-642-1
- DSSTox Substance ID: DTXSID70872897 DTXSID4047597
- MDL number MFCD00082049
- PubChem Substance ID
- InChI=1S/C3H6O.C2H4O/c1-3-2-4-3;1-2-3-1/h3H,2H2,1H3;1-2H2
- InChl Key InChI=1S/C3H6O.C2H4O/c1-3-2-4-3;1-2-3-1/h3H,2H2,1H3;1-2H2
- SMILES CC1CO1.C1CO1
- IUPAC 2-methyloxirane;oxirane
- ChEBI 32026
(1) Saxena V, Hussain MD. Poloxamer 407/TPGS mixed micelles for delivery of gambogic acid to breast and multidrug-resistant cancer. Int J Nanomedicine. 2012;7:713-21. doi: 10.2147/IJN.S28745.
Abstract. Background: Delivery of a high concentration of anticancer drugs specifically to cancer cells remains the biggest challenge for the treatment of multidrug-resistant cancer. Poloxamers and D-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS) are known inhibitors of P-glycoprotein (P-gp). Mixed micelles prepared from Poloxamer 407 and TPGS may increase the therapeutic efficacy of the drug by delivering high concentrations inside the cells and inhibiting P-gp. Gambogic acid (GA) is a naturally derived novel anticancer agent, but poor solubility and toxic side effects limit its use. In this study, we have developed Poloxamer 407 and TPGS mixed micelle-encapsulating GA for the treatment of breast and multidrug-resistant cancer.... Conclusion: This study suggests that Poloxamer 407/TPGS mixed micelles can be used as a delivery system for GA to treat breast and multidrug-resistant cancer.
(2) Raymond J, Metcalfe A, Salazkin I, Schwarz A. Temporary vascular occlusion with poloxamer 407. Biomaterials. 2004 Aug;25(18):3983-9. doi: 10.1016/j.biomaterials.2003.10.085.
Abstract. There is a need for safe and reversible occlusions during percutaneous endovascular procedures. Poloxamer 407 is a non-ionic surfactant with rapid reversible sol-gel transition behaviour. The safety and efficacy of this polymer as a temporary embolic agent was investigated. First, dissolution time after gelation of poloxamer was determined in an in vitro model. Then, transient poloxamer occlusion of renal and pulmonary arteries of seven dogs was followed by serial angiograms. Macroscopic and pathological changes were studied 1 week later. This experiment was repeated in similar arteries in one pig, and in auricular arteries of two rabbits. Poloxamer dissolution after in vitro polymerization was completed within 1-20 h, depending on concentrations. In vivo poloxamer 22% injections led to complete occlusion, followed by full recanalization within 10-90 min without complication. The only biochemical effect of poloxamer occlusions was transient elevation of triglyceride levels. There were no pathological abnormalities at 1 week. Poloxamer 407 could be used as an embolic material for temporary occlusions.
(3) Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006 Dec;23(12):2709-28. doi: 10.1007/s11095-006-9104-4.
Abstract. Poloxamer 407 copolymer (ethylene oxide and propylene oxide blocks) shows thermoreversible properties, which is of the utmost interest in optimising drug formulation (fluid state at room temperature facilitating administration and gel state above sol-gel transition temperature at body temperature promoting prolonged release of pharmacological agents). Pharmaceutical evaluation consists in determining the rheological behaviour (flow curve or oscillatory studies), sol-gel transition temperature, in vitro drug release using either synthetic or physiological membrane and (bio)adhesion characteristics. Poloxamer 407 formulations led to enhanced solubilisation of poorly water-soluble drugs and prolonged release profile for many galenic applications (e.g., oral, rectal, topical, ophthalmic, nasal and injectable preparations) but did not clearly show any relevant advantages when used alone. Combination with other excipients like Poloxamer 188 or mucoadhesive polymers promotes Poloxamer 407 action by optimising sol-gel transition temperature or increasing bioadhesive properties. Inclusion of liposomes or micro(nano)particles in Poloxamer 407 formulations offers interesting prospects, as well. Besides these promising data, Poloxamer 407 has been held responsible for lipidic profile alteration and possible renal toxicity, which compromises its development for parenteral applications. In addition, new findings have demonstrated immuno-modulation and cytotoxicity-promoting properties of Poloxamer 407 revealing significant pharmacological interest and, hence, human trials are in progress to specify these potential applications.
(4) Butt AM, Mohd Amin MC, Katas H. Synergistic effect of pH-responsive folate-functionalized poloxamer 407-TPGS-mixed micelles on targeted delivery of anticancer drugs. Int J Nanomedicine. 2015 Feb 13;10:1321-34. doi: 10.2147/IJN.S78438.
Abstract. Background: Doxorubicin (DOX), an anthracycline anticancer antibiotic, is used for treating various types of cancers. However, its use is associated with toxicity to normal cells and development of resistance due to overexpression of drug efflux pumps. Poloxamer 407 (P407) and vitamin E TPGS (D-α-tocopheryl polyethylene glycol succinate, TPGS) are widely used polymers as drug delivery carriers and excipients for enhancing the drug retention times and stability. TPGS reduces multidrug resistance, induces apoptosis, and shows selective anticancer activity against tumor cells. Keeping in view the problems, we designed a mixed micelle system encapsulating DOX comprising TPGS for its selective anticancer activity and P407 conjugated with folic acid (FA) for folate-mediated receptor targeting to cancer cells.....Conclusion: FA-P407-TPGS-DOX micelles show potential as a targeted nano-drug delivery system for DOX due to their multiple synergistic factors of selective anticancer activity, inhibition of multidrug resistance, and folate-mediated selective uptake.
(5) Bernegossi J, Calixto GM, Sanches PR, Fontana CR, Cilli EM, Garrido SS, Chorilli M. Peptide KSL-W-Loaded Mucoadhesive Liquid Crystalline Vehicle as an Alternative Treatment for Multispecies Oral Biofilm. Molecules. 2015 Dec 25;21(1):E37. doi: 10.3390/molecules21010037.
Abstract. Decapeptide KSL-W shows antibacterial activities and can be used in the oral cavity, however, it is easily degraded in aqueous solution and eliminated. Therefore, we aimed to develop liquid crystalline systems (F1 and F2) for KSL-W buccal administration to treat multispecies oral biofilms. The systems were prepared with oleic acid, polyoxypropylene (5) polyoxyethylene (20) cetyl alcohol (PPG-5-CETETH-20), and a 1% poloxamer 407 dispersion as the oil phase (OP), surfactant (S), and aqueous phase (AP), respectively. We characterized them using polarized light microscopy (PLM), small-angle X-ray scattering (SAXS), rheology, and in vitro bioadhesion, and performed in vitro biological analysis. PLM showed isotropy (F1) or anisotropy with lamellar mesophases (F2), confirmed by peak ratio quantification using SAXS. Rheological tests demonstrated that F1 exhibited Newtonian behavior but not F2, which showed a structured AP concentration-dependent system. Bioadhesion studies revealed an AP concentration-dependent increase in the system's bioadhesiveness (F2 = 15.50 ± 1.00 mN·s) to bovine teeth blocks. Antimicrobial testing revealed 100% inhibition of multispecies oral biofilm growth after KSL-W administration, which was incorporated in the F2 aqueous phase at a concentration of 1 mg/mL. Our results suggest that this system could serve as a potential vehicle for buccal administration of antibiofilm peptides.
(6) Galvis V, Tello A, Carreño NI, Berrospi RD, Niño CA. Potential use of thermoreversible hydrogel (poloxamer 407) to protect the corneal endothelium and the posterior capsule during phacoemulsification. J Cataract Refract Surg. 2019 Mar;45(3):389. doi: 10.1016/j.jcrs.2018.10.051.
(7) Singh-Joy SD, McLain VC. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics. Int J Toxicol. 2008;27 Suppl 2:93-128. doi: 10.1080/10915810802244595.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances:
Last update: 2023-05-27 12:50:44 | Chemical Risk: |