Dehydroacetic acid (3-Acetyl-4-hydroxy-6-methyl-2H-pyran-2-one) is a chemical compound, commonly derived from the condensation of ethyl acetoacetate and is produced by the reaction of acetic anhydride on acetonedicarboxylic acid. It has good stability and low volatility. It is hardly soluble in water, is thermostable and does not absorb moisture. It is an intermediate in organic synthesis.
The name describes the structure of the molecule:
- The "dehydro" part of the name indicates that it's a dehydrogenated form of acetic acid.
The synthesis process takes place in different steps:
- Preparation of Precursors. The main precursors for the synthesis of DHA are typically acetic anhydride and malonic acid.
- Condensation reaction. Precursors react together in a condensation reaction with the formation of multiple bonds and the release of water. The reaction is typically carried out at elevated temperatures.
- Cyclization. The intermediate product undergoes cyclization to form the six-member ring structure of DHA. This reaction is typically catalyzed by an acid.
- Purification. The reaction mixture is purified to isolate DHA and includes treatments such as crystallization, filtration and drying.
- Quality control test. The final product is tested to ensure it meets the required specifications. This may involve verification of purity, melting point and other physical and chemical properties.
It appears as a fine white to light yellow crystalline powder.

What it is used for and where
Medical
Basic material for the synthesis of organic compounds, in particular β-ketonols and, through interaction with aromatic aldehydes, formation of сhalcones (1).
Food
It is used commercially as an antibacterial, antifungal and preservative agent by the food industry. It is a stabiliser of vitamin C.
Although dehydroacetic acid appears on the European additives list, it cannot be used in EU countries as a food additive because it has unknown effects on human health (2).
Cosmetics
Dehydroacetic acid is a restricted ingredient as V/13 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Ingredient at risk: 3-Acetyl-6-methylpyran-2,4(3H)-dione and its salts.
- Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Used in cosmetic products such as creams and liquid soaps to prevent the growth of bacteria and moulds and acts as a photosensitiser (3).
With regard to safety assessment in cosmetic products, this study considered the endpoints of skin sensitisation, skin irritation, eye irritation, phototoxicity, acute oral toxicity, carcinogenicity, mutagenicity/genotoxicity and endocrine activity of several preservatives used in cosmetic products. These preservatives have shown a safety margin greater than 100. Dehydroacetic acid did not show a margin of safety above 100 (4).
Applications
- Cosmetic Preservative - Used in cosmetics and skincare products to prevent microbial growth and extend the product's shelf life.
- Hair Care Products - Added to shampoos, conditioners, and other hair products as a preservative.
- Food Industry - Used as a preservative in some foods, although its use is limited in many countries.
- Plastics and Resins - May be used as a stabilizer in some industrial applications.
- Pharmaceuticals - Used as a preservative in some pharmaceutical formulations.
Other uses
As an emulsion: vinyl acrylic, vinyl acetate, resin. Used in carboxymethyl cellulose, polyvinyl alcohol, starch, adhesives, incense, insect repellents.
Dehydroacetic acid studies
Caratteristiche tipiche ottimali del prodotto commerciale Dehydroacetic acid
| Appearance | White crystalline powder |
| Density | 1.3±0.1 g/cm3 1.1816 |
| Boiling Point | 270°C (1013 hPa) |
| Melting Point | 111.1 - 111.3°C |
| pH | 4 (2 g/l, H₂O, 20°C) |
Heavy Metals
| <0.0001% ≤10ppm |
Loss on drying
| ≤0.1% |
Residue on ignition
| ≤0.1% |
| pKa | 5.53±0.40 |
Refraction Index
| 1.490 |
| PSA | 60.44000 |
Vapour Pressure
| 0.0±0.7 mmHg at 25°C |
| LogP | 0.35 |
| Safety |  |
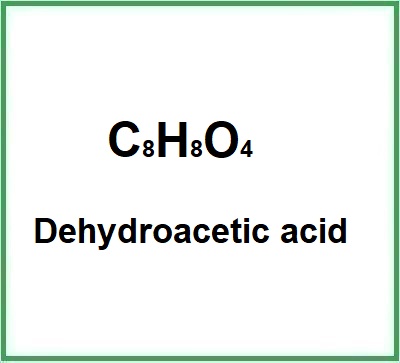
- Molecular FormulaC8H8O4
- Molecular Weight 168.148
- Exact Mass 168.042252
- CAS : 520-45-6
- UNII 2KAG279R6R
- EC Number 208-293-9
- DSSTox Substance ID DTXSID6020014
- IUPAC 3-acetyl-6-methylpyran-2,4-dione
- InChI=1S/C8H8O4/c1-4-3-6(10)7(5(2)9)8(11)12-4/h3,7H,1-2H3
- InChl Key PGRHXDWITVMQBC-UHFFFAOYSA-N
- SMILES CC1=CC(=O)C(C(=O)O1)C(=O)C
- MDL number MFCD00066709
- PubChem Substance ID 57648475
- ChEBI 137426
- SCHEMBL 17787
- NSC 760135 8770
- Beilstein 6129
- NACRES NA.22
Synonyms :
- 3-Acetyl-4-hydroxy-6-methyl-2H-pyran-2-one
- 3-Acetyl-6-Methyl-2H-Pyran-2,4(3H)-Dione
- Acetic Acid
- Dehydracetic Acid
- Methylacetopyronone
References_________________________________________________________________________
(1) Chernii S, Gerasymchuk Y, Losytskyy M, et al. Modification of insulin amyloid aggregation by Zr phthalocyanines functionalized with dehydroacetic acid derivatives. PLoS One. 2021;16(1):e0243904. Published 2021 Jan 7. doi:10.1371/journal.pone.0243904
(2) Scordino M, Lazzaro F, Borzì MA, Sabatino L, Traulo P, Gagliano G. Dehydroacetic acid in cheese and cheese coating, results of official control in Italy. Food Addit Contam Part B Surveill. 2018 Mar;11(1):75-81. doi: 10.1080/19393210.2017.1412360.
(3) Cooke MV, Chans GM, Argüello GA, Peláez WJ. Photolysis, tautomerism and conformational analysis of dehydroacetic acid and a comparison with 2-hydroxyacetophenone and 2-acetyl-1,3-cyclohexanodione. Heliyon. 2020 Jul 22;6(7):e04457. doi: 10.1016/j.heliyon.2020.e04457.
(4) Canavez ADPM, de Oliveira Prado Corrêa G, Isaac VLB, Schuck DC, Lorencini M. Integrated approaches to testing and assessment as a tool for the hazard assessment and risk characterization of cosmetic preservatives. J Appl Toxicol. 2021 Oct;41(10):1687-1699. doi: 10.1002/jat.4156.
![]() Dehydroacetic acid
Dehydroacetic acid