Ethyl Parahydroxybenzoate or Ethyl Paraben is a derivative of benzoic acid. It belongs to the Paraben family.
The name describes the structure of the molecule:
- Ethyl , Ethyl Group (C2H-5): The ethyl group is a functional group derived from ethane (C2H6). It consists of two carbon atoms and five hydrogen atoms. When a hydrogen atom is removed from the ethane, an ethyl group is formed, which can form a bond with other atoms or groups of atoms. In the context of ethyl paraben, the ethyl group is bonded to the oxygen atom of the carboxylic group of p-hydroxybenzoic acid, forming an ester bond.
- Parahydroxybenzoate. Derived from p-hydroxybenzoic acid, which is a type of paraben. Parabens are a class of preservatives widely used in cosmetic and pharmaceutical products. The structure of p-hydroxybenzoic acid consists of a benzene ring (a hexagonal ring of six carbon atoms) to which a carboxylic group (-COOH) and a hydroxyl group (-OH) are bonded. In the para configuration, these two groups are attached to opposite sides of the benzene ring. When the carboxylic group of p-hydroxybenzoic acid reacts with ethanol during esterification, it forms the ester known as ethyl paraben.
The synthesis process takes place in different steps:
- Production. The first step involves the production of parahydroxybenzoic acid, which generally results from a reaction between phenol and carbon dioxide in a process known as the Kolbe-Schmitt reaction.
- Esterification. Parahydroxybenzoic acid enters into a reaction with ethanol. In this esterification process, the carboxylic group (COOH) of parahydroxybenzoic acid reacts with the hydroxyl group (OH) of ethanol to form an ester, releasing water as a by-product. The resulting compound is ethyl parahydroxybenzoate.
What is it for?
Food
Ingredient included in the list of European food additives as E215, an additive with a preservative and bactericidal function used in various fields including the cosmetic one and used to counteract bacteria, fungi, yeasts and molds.
Safety
Parabens are preservative chemical compounds that have been the subject of attention in the scientific literature as possible endocrine disruptors (particularly propylparaben and butylparaben), i.e. with the possibility of damaging the hormone-producing glands in our bodies, particularly in the breasts. The 2004 study by Darbre et al. showed that parabens remain in our bodies as intact esters (2). Following this study, some of the scientific literature in 2005 and 2006 cast doubt on Darbre's conclusions and claimed they were limited. However, both the US FDA and the European SCCP authorised in 2006 the use of a single paraben in cosmetic products at a concentration of 0.4% and the use of total parabens at a concentration of 0.8%. However, there is no shortage of studies that consider the restrictions unnecessary: M. G. Kirchhof et al. in 2013 found that parabens are among the safest and most well-tolerated preservatives and that current data do not support drastic regulations or personal exposure restrictions. Darbre in 2014 published a further study in which he showed how parabens can cause DNA damage.
11-6-2019 I wrote to the European Commission's Directorate for Health and Food Safety (DG SANTE) reiterating doubts about the safety of parabens. Finally, also from this body came the answer that clarifies all doubts:
"Regarding the use of methyl- and propylparaben as excipients in oral medicinal products for human use, I would advise you to look at the information provided by the European Medicines Agency at https://www.ema.europa.eu/en/use-methyl-propylparaben-excipients-human-medicinal-products-oral-use This discussion paper deals with methyl- and propylparaben, as these are the parabens predominantly used in oral pharmaceutical formulations. The focus of this paper is on possible endocrine disrupting effects in humans."
Parabens are components discussed and on whose safety many doubts have been raised, especially for the damage they would produce to the aquatic environment where they are discharged after use (1).
Parabens can contribute to obesity (2).

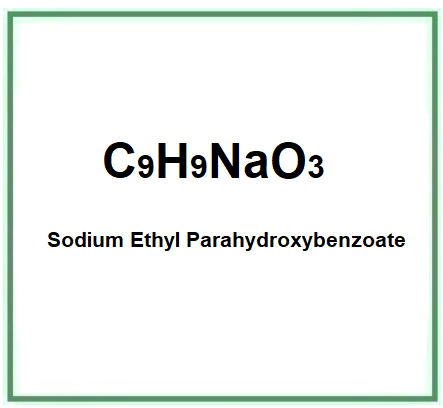
- Molecular Formula C9H9NaO3 C9H9O3Na
- Molecular Weight 188.16 g/mol
- CAS 35285-68-8
- UNII Z0D00IVA10
- EC number 252-487-6
- Nikkaji Number J42.047F
Synonyms :
Sodium ethylparaben
sodium;4-ethoxycarbonylphenolate
Sodium Ethyl Parahydroxybenzoate
Benzoic acid, 4-hydroxy-, ethyl ester, sodium salt
4-Hydroxybenzoic acid, ethyl ester, sodium salt
Ethylparaben sodium (NF)
Ethylparaben, sodium salt
EINECS 252-487-6
Ethyl p-hydroxybenzoate, sodium salt
Sodium 4-ethoxycarbonylphenoxide
Sodium 4-(ethoxycarbonyl)phenolate
References_______________________________________________________________________
(1) Terasaki M, Abe R, Makino M, Tatarazako N. Chronic toxicity of parabens and their chlorinated by-products in Ceriodaphnia dubia. Environ Toxicol. 2013 Dec 27. doi: 10.1002/tox.21944. [Epub ahead of print]
Popa DS, Bolfa P, Kiss B, Vlase L, Păltinean R, Pop A, Cătoi C, Crişan G, Loghin F.
Influence of Genista tinctoria L. or methylparaben on subchronic toxicity of bisphenol A in rats. Biomed Environ Sci. 2014 Feb;27(2):85-96. doi: 10.3967/bes2014.021.
(2) Hu P, Chen X, Whitener RJ, Boder ET, Jones JO, Porollo A, Chen J, Zhao L. Effects of parabens on adipocyte differentiation. Toxicol Sci. 2013 Jan;131(1):56-70. doi: 10.1093/toxsci/kfs262. Epub 2012 Sep 5.
![]() Sodium Ethyl Parahydroxybenzoate
Sodium Ethyl Parahydroxybenzoate