![]() Sodium croscarmellose
Sodium croscarmellose
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Avoid excessive amounts (1)8 pts from AColumn
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Croscarmellose sodium insights" about Sodium croscarmellose Review Consensus 8 by AColumn (9336 pt) | 2023-Jul-26 20:07 |
| Read the full Tiiip | (Send your comment) |
Updates concern efficacy, melting time, possible allergic reactions, drug interactions, formulations.
Bindra DS, Stein D, Pandey P, Barbour N. Incompatibility of croscarmellose sodium with alkaline excipients in a tablet formulation. Pharm Dev Technol. 2014;19(3):285–289. doi:10.3109/10837450.2013.778869
Abstract. The objective of the current work was to study an observed incompatibility between croscarmellose sodium and basic excipients in a tablet formulation. Significant dissolution slowdown was observed for alkaline tablet compositions of an acid-labile drug containing croscarmellose sodium (CCS) as a disintegrant. The severity of the dissolution slowdown was directly proportional to both the degree of alkalinity and the level of CCS in the tablet formulation. It is postulated that the ester cross-links in CCS were partially or fully hydrolyzed under basic conditions (pH values >9) forming by-products of increased water solubility. This increase in the level of water-soluble polymer can lead to the formation of a viscous barrier in the tablet upon moisture uptake, thus slowing down its dissolution. The dissolution slowdown was not observed for a similar alkaline tablet preparation containing crospovidone as a disintegrant.
Eraga SO, Arhewoh MI, Akpan FE, Iwuagwu MA. Evaluation of fast disintegrating tablets of paracetamol prepared from a blend of croscarmellose sodium and Pleurotus tuber-regium powder. Pak J Pharm Sci. 2018;31(6):2503–2508.
Abstract. The study investigated the combination effects of the mixture of croscarmellose sodium and Pleurotus tuber-regium powder on the granules and tableting parameters of paracetamol tablets. Five batches (A-E) of paracetamol tablets were formulated using wet granulation method with various combination ratios of croscarmellose sodium and Pleurotus tuber-regium powder as disintegrant incorporated both intra- and extra granularly. Their granule properties such as bulk and tapped densities, angle of repose, Carr's index, Hausner's ratio and post compression parameters such as friability, hardness, disintegration time and drug release profiles were evaluated. The results showed a decrease in disintegration time with increasing concentration of Pleurotus tuber-regium powder with disintegration times < 3.58 min. There was an increase in hardness (values > 4.34 kp) and a decrease in friability (values < 0.6 %) of the tablets with increasing concentrations of Pleurotus tuber-regium. All the tablets exhibited comparable drug release profiles with over 80 % of their drugs released in 1 h. Harder and less friable fast disintegrating tablets of paracetamol can be obtained with Pleurotus tuber-regium powder in combination with croscarmellose sodium. The combination of croscarmellose sodium and Pleurotus tuber-regium possesses potentiative effect on their disintegrant activity.
Zhao N, Augsburger LL. The influence of product brand-to-brand variability on superdisintegrant Performance. A case study with croscarmellose sodium. Pharm Dev Technol. 2006;11(2):179–185. doi:10.1080/10837450600561281
Abstract. The purpose of this study is to investigate factors influencing croscarmellose sodium functionality with special emphasis on developing a discriminating model tablet formulation to evaluate product brand-to-brand variability. The particle size distribution, water uptake, and swelling properties of five brands of croscarmellose sodium in either neutral water or 0.1 N HCl were studied. Differences were observed in all properties between brands. Media with acidic pH had a negative impact, but to different extents, on both the water uptake and swelling of all croscarmellose sodium brands due to the presence of carboxymethyl sodium substituents. A tablet matrix composed of lactose (75% w/w) and dicalcium phosphate (25% wt/wt) was used to compare the functional equivalency of the five brands of croscarmellose sodium. The tablet disintegration times were inversely proportional to the swelling ability of superdisintegrant in the testing medium regardless of medium temperature and disintegrant concentration. In conclusion; the particle size, total degree of substitution, and the ratio of basic to acidic substituents are important factors that should be considered during product optimization. The tablet matrix composed of lactose and dicalcium phosphate at a weight ratio of 3:1 can be used as a model formulation for product lot-to-lot consistency and product brand-to-brand comparison purposes.
Larsen J, Melander C. Study of interaction between croscarmellose and escitalopram during sample preparation. Drug Dev Ind Pharm. 2012;38(10):1195–1199. doi:10.3109/03639045.2011.643896
Abstract. During routine analysis of an escitalopram tablet formulation, it was seen that there was a systematic deviation between content uniformity (CU - one tablet analysis) and assay analysis (ten pooled tablets). In the presence of the excipients from the tablet, it was found that the extraction of the active pharmaceutical ingredient (API) was incomplete. It was shown that the commonly used tablet disintegrant croscarmellose sodium (crosslinked carboxy-methyl cellulose) had a significant interaction with escitalopram. This was later found to be the explanation for the lower extraction during assay testing. Under normal conditions, the extraction took place in acidic medium which caused protonation of the amine and thereby the interaction of charged species in solution. The interaction of API was studied further with pure croscarmellose and the entire tablet matrix. A range of conditions was considered, including altering extraction volumes, organic solvents, pH of the extraction solvent, and addition of competitive binder in various concentrations. It was seen that arginine was the most effective cationic competitive binder of those tested and that adding it at a suitable concentration level could significantly improve the analytical methods. In the present case, an improvement in recovery was from 98.5% to almost 100% was achieved.
Faroongsarng D, Peck GE. Thermal porosity analysis of croscarmellose sodium and sodium starch glycolate by differential scanning calorimetry. AAPS PharmSciTech. 2003;4(4):E67. Published 2003 Dec 30. doi:10.1208/pt040467
Abstract. The aim of the study was to demonstrate the applicability of differential scanning calorimetry (DSC) on porosity analysis for cellulose and starch. Croscarmellose sodium (CCS) and sodium starch glycolate (SSG) were allowed to sorb moisture in 85%, 90%, 95%, and 100% relative humidity (RH) at 40 degrees C for 24 hours. The pretreated samples were then subjected to DSC running temperature ranging from 25 degrees C to -50 degrees C at a cooling rate of 10 degrees C/min. The cooling traces of water crystallization, if present, were transformed to porosity distribution via capillary condensation using Kelvin's equation. The porosity analysis of CCS and SSG was also done using nitrogen adsorption as a reference method. It was found that sorbed water could not be frozen (in cases of 85% and 90% RH) until the moisture content exceeded a cutoff value (in cases of 95% and 100% RH). The nonfreezable moisture content was referred to tightly bound, plasticizing water, whereas the frozen one may be attributed to loosely bound water condensation in pore structure of CCS and SSG surfaces. Not only capillary condensation but also the tightly bound, nonfreezable monolayer water lying along the inner pores of the surface contributed to porosity determination. Good agreement with less than 5% deviation of mean pore size was observed when the results were compared with nitrogen adsorption. The narrower pore size distributions, however, were obtained because of the limitations of the technique. It was concluded that pore analysis by DSC could be successful. Further research needs to be done to account for limitations and to extend the applicability of the technique.
Mumoli N, Cei M, Luschi R, Carmignani G, Camaiti A. Allergic reaction to Croscarmellose sodium used as excipient of a generic drug. QJM. 2011;104(8):709–710. doi:10.1093/qjmed/hcq175
Jadhav YG, Galgatte UC, Chaudhari PD. Overcoming Poor Solubility of Dimenhydrinate: Development, Optimization and Evaluation of Fast Dissolving Oral Film. Adv Pharm Bull. 2018;8(4):721–725. doi:10.15171/apb.2018.081
Abstract. Purpose: To develop fast dissolving oral film to address vomiting and nausea in pediatric population. Methods: Oral films of Dimenhydrinate were prepared by solvent casting method by using hydroxypropylmethyl cellulose E5 (HPMC E5), polyethylene glycol 400 (PEG 400) and croscarmellose sodium. Solubility of dimenhydrinate was enhanced by ethanol as a co-solvent. To make dimenhydrinate palatable sodium saccharin and peppermint oil were used. All films were evaluated for mechanical parameters, surface pH, morphology, disintegration time and percent dissolution. Results: Films were smooth, acceptable and white in colour. For optimized batch, drug content (99.106%), disintegration time (25 sec), dissolution (99.10% in 210 sec), surface pH (6.81) were acceptable. Conclusion: Optimized batch, due to its potential to deliver through fast dissolving film, can be developed for clinical use.
Berardi A, Bisharat L, Blaibleh A, Pavoni L, Cespi M. A Simple and Inexpensive Image Analysis Technique to Study the Effect of Disintegrants Concentration and Diluents Type on Disintegration. J Pharm Sci. 2018;107(10):2643–2652. doi:10.1016/j.xphs.2018.06.008
Domosławska M, Pawlak-Morka R, Dobrzyński Ł, Herda M. Study of the influence of cellulose derivatives on physical and analytical attributes of a drug product belonging to BCS class II. Polim Med. 2018;48(2):83–90. doi:10.17219/pim/104462
Abstract. Background: Cellulose microcrystalline (MCC), hydroxypropyl methylcellulose (HPMC) and croscarmellose sodium are cellulose derivatives which are widely used in pharmaceutical technology. Although they are inert pharmaceutical ingredients, they can influence the release profile of an active substance from the dosage form depending on their distribution, type and quantity used in the formulation. Objectives: The aim of the present investigation was to examine the effect of chosen cellulose derivatives on the physical and analytical attributes of a drug product containing an active substance of Biopharmaceutics Classification System (BCS) class II. Material and methods: The tablets were prepared using the wet granulation technology. The batches differed in the amount and grade of HPMC, the type of MCC and the distribution of croscarmellose sodium. The granule properties as well as physical (tablet hardness, disintegration time, friability) and analytical (dissolution profile in different media) attributes of the tablets were examined. Results: The flow characteristics were satisfying in the case of all prepared batches. However, the differences in flow properties were visible, especially in the cases where MCC of coarser particles was replaced with MCC of finer particles. The type of MCC used in the product formula also had a significant influence on the drug product dissolution profile. The batches in which MCC of finer particles was used had substantially better results, regardless of HPMC viscosity type and the distribution of croscarmellose sodium between the inner and outer phase. What is more, the differences in the results between batches of different MCC types were especially visible in dissolution conditions, i.e., 0.1N hydrochloric acid (HCl). Conclusions: By choosing the right type, quantity and distribution of cellulose derivatives, it was possible to obtain the optimal formula of the drug product similar to in-vitro conditions to the reference drug. Out of all the tested excipients, the type of cellulose microcrystalline was found to have the most critical influence on both physical and analytical properties of the pharmaceutical formulation.
Kwak SS, Lee ES, Yoon HY, et al. Immediate release tablet formulation of varenicline salicylate and comparative pharmacokinetic study in human volunteers. Drug Des Devel Ther. 2018;12:3377–3392. Published 2018 Oct 9. doi:10.2147/DDDT.S178456
Abstract. Purpose: To develop an immediate release-type tablet containing varenicline salicylate (VRC-S), a smoking cessation agent, formulation and stability studies were performed. The in vitro dissolution and in vivo pharmacokinetic (PK) behavior of the tablets were compared with those of the commercial product (Champix) as a reference. Materials and methods: The characteristics of the powder were investigated by particle morphology, size distribution, solubility, hygroscopicity, differential scanning calorimetry, and powder X-ray diffraction. Based on the drug-excipient compatibility test, different VRC-S tablets were prepared with the selected excipients through direct compression or wet granulation method and subjected to a dissolution test. The stability of the most promising VRC-S tablet (F4) was evaluated under accelerated conditions (40°C and 75% relative humidity). Further, the dissolution and human pharmacokinetic profiles of the F4 tablet and Champix were compared. Results: VRC-S showed a positively skewed unimodal size distribution with a specific surface area of 2.02 m2/g, single endothermic peak of 225.2°C in differential scanning calorimetry, crystalline internal structure in powder X-ray diffraction, aqueous solubility of 244.7 mg/mL, and hygroscopicity of 0.256 mg/g. The wet granulation method was preferred for tablet preparation and employed the following excipients: microcrystalline cellulose and anhydrous dibasic calcium phosphate as diluents, croscarmellose sodium as a disintegrant, and colloidal silicon dioxide and magnesium stearate as lubricants. The F4 tablet was stable for 6 months under accelerated conditions. The dissolution of VRC was pH independent, revealing f 2 values of 76.49 and 68.38 at pH 1.2 and pH 6.8, respectively. After the oral administration of F4 tablet and Champix to healthy human volunteers, pharmacokinetic parameters, including time to reach the maximum plasma concentration (Tmax), maximum plasma concentration (Cmax), and area under the curve from 0 to infinity (AUCinf), were compared. The values of 90% CI were 0.972-1.035 for Cmax and 0.982-1.075 for AUCinf, which was indicative of the bioequivalence of both products.
Kumar MU, Babu MK. Design and evaluation of fast dissolving tablets containing diclofenac sodium using fenugreek gum as a natural superdisintegrant. Asian Pac J Trop Biomed. 2014;4(Suppl 1):S329–S334. doi:10.12980/APJTB.4.2014B672
Abstract. Objective: To formulate diclofenac sodium as fast dissolving tablets (FDTs) using fenugreek gum as a natural superdisintegrant which also possess anti-inflammatory activity. Methods: An attempt was made to extract the fenugreek gum and evaluated it for various physicochemical characterizations. The swelling index and viscosity of fenugreek gum was 221% and 293.4 mpa.s respectively. FDTs of diclofenac sodium was formulated by direct compression technique using different concentrations (1%-6%, w/w) of fenugreek gum as a natural superdisintegrant and compared with renowned synthetic superdisintegrants like sodium starch glycolate and croscarmellose sodium. The anti-inflammatory activity of a formulation was evaluated with carrageenan induced experimental rats. Results: The formulated tablets were evaluated for various physical tests like weight variation, friability, hardness and results complied with the limits. The drug release from all the formulations ascertained first order kinetics. Among all the formulations F3 containing fenugreek gum with the concentration of 6% produced least disintegrating time 21 seconds resulting in higher drug release rate 93.74% at the end of 25 min. Hence, it was considered as optimized formulation. The present study revealed that the fenugreek gum as a natural superdisintegrant showed better disintegrating property than the most widely used synthetic superdisintegrants like sodium starch glycolate and croscarmellose sodium in the formulations of FDTs. Conclusions: The results suggested that the fenugreek gum act as a good super disintegrating agent and it showed promising additive anti-inflammatory activity with diclofenac sodium.
Bolko Seljak K, Ilić IG, Gašperlin M, Zvonar Pobirk A. Self-microemulsifying tablets prepared by direct compression for improved resveratrol delivery. Int J Pharm. 2018;548(1):263–275. doi:10.1016/j.ijpharm.2018.06.065
Abstract. The purpose of this study was to develop self-microemulsifying (SME-) tablets to improve resveratrol solubility whilst delivering resveratrol in a preferred tablet dosage form. Resveratrol was dissolved in liquid self-microemulsifying drug delivery system (SMEDDS) (10% w/w) and solidified through adsorption on several different solid carriers. Two ranges of synthetic amorphous silica (Sylysia® 290, 350, 470, 580; Syloid® 244FP, AL-1FP) as well as granulated magnesium aluminometasilicate (Neusilin® US2) were screened for their SMEDDS adsorbent capacity. The most efficient carrier from every range was chosen for further SME-tablet development. To counteract the high ratio of liquid in SME-tablets, additional dry binders (microcrystalline cellulose, copovidone) were added to the tableting mixture, as well as superdisintegrant (croscarmellose sodium) and lubricant (magnesium stearate). Finally, approx. 600 mg tablets were directly pressed using 12 mm flat face punch, containing 41.75% SMEDDS. Overall, all tablets exhibited sufficient hardness (>50 N), although it was negatively affected by higher compression force. Tablets with Neusilin® US2 proved to have highest hardness, as granulated structure of Neusilin® US2 provided best compaction properties needed for successful direct compression of tablets. All prepared SME tablet formulations disintegrated in under 10 min and formed microemulsions (droplet size < 100 nm) upon dilution with water, with Neusilin® US2 tablets exhibiting the lowest droplet size (<30 nm). While conventional dissolution test indicated incomplete resveratrol release from solid carriers in both pH 1.2 and 6.8 media, no difference fatty acid amount titrated during fasted state in vitro lipolysis between liquid and solid SMEDDS was observed. Moreover, accelerated stability tests confirmed over 90% of trans-resveratrol remained in solid SMEDDS following 90 days at 40 °C, with no crystallization of resveratrol observed during that time. To sum up, through adsorption on solid carriers, in particular Neusilin® US2, SMEDDS was successfully transformed into a directly compressible mixture and tableted without the loss of its self-microemulsifying ability.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Sodium croscarmellose Review Consensus 8 by AColumn (9336 pt) | 2024-Oct-11 18:23 |
| Read the full Tiiip | (Send your comment) |
Croscarmellose Sodium (E468, CNC) is a cross-linked sodium salt of carboxymethyl cellulose, commonly used in pharmaceutical formulations as a superdisintegrant. Its primary function is to facilitate the rapid breakdown of tablets, capsules, or other solid dosage forms in the digestive system, allowing the active ingredients to dissolve more quickly and be absorbed efficiently. It is also used in some cosmetic and personal care products as a thickening or stabilizing agent.
Chemical Composition and Structure
Sodium croscarmellose is derived from cellulose, which is chemically modified by introducing carboxymethyl groups. These groups are then cross-linked to form a network that expands when in contact with water, making it an excellent disintegrant. Its cross-linked structure enhances water absorption, which in turn promotes rapid tablet disintegration.
Physical Properties
Sodium croscarmellose is typically a white to off-white, odorless, and free-flowing powder. It is water-insoluble but swells rapidly upon contact with water, making it ideal for use as a disintegrant in pharmaceutical tablets and capsules. The swelling action helps break down solid formulations into smaller particles for faster dissolution and absorption of active ingredients.
The name describes the structure of the molecule:
- Croscarmellose refers to a carboxymethyl cellulose that has been cross-linked, i.e. its polymer chains are linked to form a three-dimensional network. Carboxymethyl cellulose is a derivative of cellulose, the main structural component of plant cell walls, which has been modified by the addition of carboxymethyl groups (-CH2-COOH).
- sodium refers to the sodium ions (Na+) associated with the carboxymethyl groups of the polymer. When carboxymethylcellulose is cross-linked, the hydrogen ions (H+) in the carboxy groups (-COOH) are replaced by sodium ions, forming the sodium salt of carboxymethylcellulose, or croscarmellose sodium.
The synthesis process generally involves the reaction of cellulose, a natural polymer derived from plant materials, with a cross-linking agent in an alkaline solution. The cross-linking agent, usually sodium monochloroacetate, reacts with cellulose to introduce carboxymethyl groups, creating carboxymethyl cellulose. This is further cross-linked to create sodium croscarmellose, which has the unique property of swelling rapidly when in contact with water.
In practice:
- Step 1: Preparation of raw materials. The raw materials for the synthesis of croscarmellose sodium are typically cellulose and sodium hydroxide.
- Step 2: Carboxymethylation. The cellulose is reacted with sodium hydroxide and chloroacetic acid to introduce carboxymethyl groups on the spine of the cellulose, creating carboxymethylcellulose.
- Step 3: Crosslinking. The carboxymethylcellulose is then crossed to form croscarmellose. This is typically done using a crosslinking agent such as epichlorohydrin.
- Step 4: Purification and drying. The resulting croscarmellose sodium is then purified, typically through filtration and washing, and dried.
It occurs as a fine white, fibrous, odourless powder. It is insoluble in ether, ethanol, acetone or toluene. In contact with water, the volume expands 4 to 8 times its original volume.

What it is used for and where
Medical and Pharmaceuticals
It is an emulsifying disintegrant formed from crude cellulose treated with sodium compounds. It is used to facilitate the breaking up of tablets to improve and accelerate the release of the drug in the intestine after oral administration.
Together with crospovidone, sodium starch glycolate, and others, it is considered a superdisintegrant (1).
Tablets with the same total concentration of superdisintegrant dissolve at a faster rate when the superdisintegrant is included intragranularly (2).
The results of this study demonstrate the low toxicity and safe use of Croscarmellose sodium in oral applications such as pharmaceuticals, dietary supplements, and sweetener tablets (3).
Food
Labelled as E468 in the list of European thickener additives. Food thickeners are normally used to facilitate the ingestion of drugs in tablet form. Some thickeners directly influence the dissolution and disintegration of tablets and may even delay their dissolution.
Excessive intake of celluloses such as E468 may be associated with high risks of cardiovascular diseases (CVD).(4)
Typical optimal characteristics of CROSCARMELLOSE SODIUM as commercial product
| Appearance | White free following powder odorless |
| Grade | Ph.Eur.,JP,NF |
| pH | 5.0~7.0 (10 g/l, H₂O, 20 °C) |
| Density bulk | 0.4 kg/m3 |
| Solubility | 30 g/l |
| Na-chloride&Na-glycolate | ≤0.50% |
| Degree of substitution | 0.60~0.80 |
| Heavy metals | ≤10ppm |
| Water soluble substances | <9 |
| Setting volume | 10~30 |
| Ash | 14.0~26% |
| Storage | +2°C e +30°C. |
CROSCARMELLOSE SODIUM
 |  |
CROSCARMELLOSE
 |  |
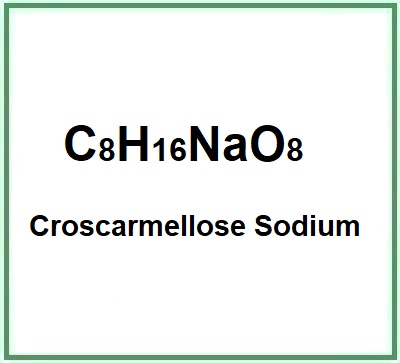
- Molecular Formula C8H16NaO8
- Molecular Weight 263.20
- CAS:74811-65-7 9004-32-4 9000-11-7
- UNII M28OL1HH48
- EC Number: 618-326-2 618-378-6
- DSSTox Substance ID: DTXSID2020555
- InChI=1S/C6H13NO/c1-2-6-5-8-4-3-7-6/h6-7H,2-5H2,1H3/t6-/m0/s1
- SMILES O1C([H])([H])C([H])([H])N([H])[C@@]([H])(C([H])([H])C([H])([H])[H])C1([H])[H]
- MDL number
- PubChem Substance ID
Synonyms:
- Croscarmellose Sodium
- CNC
- Carboxymethylcellulose Sodium
- Carboxymethylcellulose
- Cellulose, Carboxymethyl
- Cross-linked carboxymethylcellulose sodium
References__________________________________________________________________
(1) Yousaf AM, Naheed F, Shahzad Y, Hussain T, Mahmood T. Influence of sodium starch glycolate, croscarmellose sodium and crospovidone on disintegration and dissolution of stevia-loaded tablets. Polim Med. 2019;49(1):19–26. doi:10.17219/pim/111516
Abstract. Background: Sugar substitutes are used by diabetic, obese and calorie-conscious people. As artificial sweeteners are harmful to the body, natural sweeteners are more suitable. Sugar substitutes are available on the market in tablet forms, which are added to hot or cold drinks. Rapid disintegration and dissolution of sugar substitute-loaded tablet is desired. However, the tablets should be hard enough to maintain their integrity during mechanical shocks. Objectives: The objective of this research was to develop rapidly disintegrating and dissolving stevia-loaded tablets with appropriate wetting, hardness and friability....Conclusions: The tablet consisting of stevia, crospovidone, lactose, and magnesium stearate at the weight ratio of 15/2.5/32/0.5 showed excellent results with regards to dissolution and disintegration; accordingly, this formulation could be a potential sugar substitute for diabetic, obese and/or calorie-conscious individuals.
(2) Gordon MS, Chatterjee B, Chowhan ZT. Effect of the mode of croscarmellose sodium incorporation on tablet dissolution and friability. J Pharm Sci. 1990;79(1):43–47. doi:10.1002/jps.2600790111
Abstract. A computer-optimized experimental design was used to study the effect of incorporating a "super disintegrant", croscarmellose sodium, intragranularly, extragranularly, or distributed equally between the two phases of a tablet in which a poorly soluble drug constituted at least 92.5% of the formulation. The results were analyzed by means of a general quadratic response surface model and suggest that tablets with the same total concentration of super disintegrant dissolve at a faster rate when the super disintegrant is included intragranularly. Tablet friability was not affected by the method of super disintegrant incorporation.
(3) Freeman C, Weiner ML, Kotkoskie LA, Borzelleca J, Butt M. Subchronic and developmental toxicity studies in rats with Ac-Di-Sol croscarmellose sodium. Int J Toxicol. 2003;22(3):149–157. doi:10.1080/10915810305108
Abstract. Studies were conducted to evaluate the subchronic and developmental toxicity of Ac-Di-Sol (croscarmellose sodium). In the subchronic study, groups of Sprague-Dawley rats (20/sex/group) received 0 (control), 10000, or 50000 ppm Ac-Di-Sol in the diet for 90 consecutive days (equivalent to 757 and 893 mg/kg/day for males and females fed 10000 ppm, respectively, and to 3922 and 4721 mg/kg/day for males and females fed 50000 ppm, respectively). No mortality, clinical signs of toxicity, or adverse toxicological effects on hematology or serum chemistry parameters, feed consumption, or ophthalmologic examinations were noted in any treatment group. Body weight gain was depressed in high-dose males during the final 3 weeks. The only treatment-related histological lesion noted was moderate renal mineralization at the corticomedullary junction in one high-dose female. This lesion was not considered a specific effect of Ac-Di-Sol, but rather a secondary effect resulting from a potential increase in urinary pH and renal excretion of sodium due to the high intake of sodium associated with Ac-Di-Sol. In the developmental toxicity study, groups of pregnant Sprague-Dawley rats (25/group) received 0 (control), 10000, or 50000 ppm Ac-Di-Sol in the diet on gestational days 6 to 15. No evidence of maternal, fetal, or embryo toxicity was noted. The no-observed-adverse-effect level (NOAEL) for Ac-Di-Sol in both studies exceeds 50000 ppm in the diet, which represents doses of 3922 and 4712 mg/kg/day, for males and females, respectively. The results of these studies demonstrate the low subchronic oral toxicity and developmental toxicity of Ac-Di-Sol, and support the safe use of Ac-Di-Sol in oral applications such as pharmaceuticals, dietary supplements, and sweetener tablets.
(4) Sellem L, Srour B, Javaux G, Chazelas E, Chassaing B, Viennois E, Debras C, Salamé C, Druesne-Pecollo N, Esseddik Y, de Edelenyi FS, Agaësse C, De Sa A, Lutchia R, Louveau E, Huybrechts I, Pierre F, Coumoul X, Fezeu LK, Julia C, Kesse-Guyot E, Allès B, Galan P, Hercberg S, Deschasaux-Tanguy M, Touvier M. Food additive emulsifiers and risk of cardiovascular disease in the NutriNet-Santé cohort: prospective cohort study. BMJ. 2023 Sep 6;382:e076058. doi: 10.1136/bmj-2023-076058. PMID: 37673430; PMCID: PMC10480690.
Arca HC, Mosquera-Giraldo LI, Bi V, Xu D, Taylor LS, Edgar KJ. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules. 2018 Jul 9;19(7):2351-2376. doi: 10.1021/acs.biomac.8b00517.
Abstract. Cellulose ethers have proven to be highly useful natural-based polymers, finding application in areas including food, personal care products, oil field chemicals, construction, paper, adhesives, and textiles. They have particular value in pharmaceutical applications due to characteristics including high glass transition temperatures, high chemical and photochemical stability, solubility, limited crystallinity, hydrogen bonding capability, and low toxicity. With regard to toxicity, cellulose ethers have essentially no ability to permeate through gastrointestinal enterocytes and many are already in formulations approved by the U.S. Food and Drug Administration. We review pharmaceutical applications of these valuable polymers from a structure-property-function perspective, discussing each important commercial cellulose ether class; carboxymethyl cellulose, methyl cellulose, hydroxypropylcellulose, hydroxypropyl methyl cellulose, and ethyl cellulose, and cellulose ether esters including hydroxypropyl methyl cellulose acetate succinate and carboxymethyl cellulose acetate butyrate. We also summarize their syntheses, basic material properties, and key pharmaceutical applications.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-08-13 16:22:42 | Chemical Risk: |