Check the ingredients!
... live healthy!


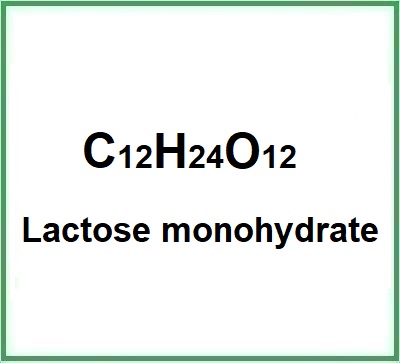
![]() Lactose monohydrate
Lactose monohydrate
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Specific allergy (1)7 pts from FRanier
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Lactose monohydrate insights" about Lactose monohydrate Review Consensus 8 by FRanier (9976 pt) | 2020-Apr-19 15:57 |
| Read the full Tiiip | (Send your comment) |
In-depth studies on Lactose monohydrate include the latest studies on activity, compatibility with other chemical compounds, indications and contraindications.
Makraduli L, Makreski P, Goracinova K, Stefov S, Anevska M, Geskovski N. A Comparative Approach to Screen the Capability of Raman and Infrared (Mid- and Near-) Spectroscopy for Quantification of Low-Active Pharmaceutical Ingredient Content Solid Dosage Forms: The Case of Alprazolam [published online ahead of print, 2020 Apr 9]. Appl Spectrosc. 2020;3702820905367. doi:10.1177/0003702820905367
Gajjar P, Styliari ID, Nguyen TTH, et al. 3D Characterisation of Dry Powder Inhaler Formulations: Developing X-ray Micro Computed Tomography Approaches [published online ahead of print, 2020 Apr 5]. Eur J Pharm Biopharm. 2020;S0939-6411(20)30054-0. doi:10.1016/j.ejpb.2020.02.013
Rahman Z, Dharani S, Barakh Ali SF, Nutan MTH, Khan MA. Effects of Diluents on Physical and Chemical Stability of Phenytoin and Phenytoin Sodium. AAPS PharmSciTech. 2020;21(3):104. Published 2020 Mar 12. doi:10.1208/s12249-020-1639-x
Salústio PJ, Machado M, Nunes T, Sousa E Silva JP, Costa PC. Lactose monohydrate flow characterization using shear cell method [published online ahead of print, 2020 Feb 27]. Pharm Dev Technol. 2020;1–8. doi:10.1080/10837450.2020.1731531
McDonagh AF, Tajber L. Crystallo-co-spray drying as a new approach to manufacturing of drug/excipient agglomerates: Impact of processing on the properties of paracetamol and lactose mixtures. Int J Pharm. 2020;577:119051. doi:10.1016/j.ijpharm.2020.119051
Murphy EG, Regost NE, Roos YH, Fenelon MA. Powder and Reconstituted Properties of Commercial Infant and Follow-On Formulas. Foods. 2020;9(1):84. Published 2020 Jan 13. doi:10.3390/foods9010084
Veras KS, Fachel FNS, Pittol V, et al. Compatibility study of rosmarinic acid with excipients used in pharmaceutical solid dosage forms using thermal and non-thermal techniques. Saudi Pharm J. 2019;27(8):1138–1145. doi:10.1016/j.jsps.2019.09.010
Skelbæk-Pedersen AL, Vilhelmsen TK, Wallaert V, Rantanen J. Investigation of the effects of particle size on fragmentation during tableting. Int J Pharm. 2020;576:118985. doi:10.1016/j.ijpharm.2019.118985
Pazesh S, Persson AS, Alderborn G. Atypical compaction behaviour of disordered lactose explained by a shift in type of compact fracture pattern. Int J Pharm X. 2019;1:100037. Published 2019 Nov 8. doi:10.1016/j.ijpx.2019.100037
Ribeiro AB, de Araújo CB, Silva LEV, et al. Hygiene protocols for the treatment of denture-related stomatitis: local and systemic parameters analysis - a randomized, double-blind trial protocol. Trials. 2019;20(1):661. Published 2019 Nov 29. doi:10.1186/s13063-019-3854-x
Della Bella A, Müller M, Danani A, Soldati L, Bettini R. Effect of Lactose Pseudopolymorphic Transition on the Aerosolization Performance of Drug/Carrier Mixtures. Pharmaceutics. 2019;11(11):576. Published 2019 Nov 4. doi:10.3390/pharmaceutics11110576
Salústio PJ, Machado M, Nunes T, Sousa E Silva JP, Costa PC. Lactose monohydrate flow characterization using shear cell method [published online ahead of print, 2020 Feb 27]. Pharm Dev Technol. 2020;1–8. doi:10.1080/10837450.2020.1731531
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Lactose monohydrate Review Consensus 7 by FRanier (9976 pt) | 2024-Oct-02 11:47 |
| Read the full Tiiip | (Send your comment) |
Lactose monohydrate is a naturally occurring disaccharide sugar that is derived from milk. It consists of one glucose molecule and one galactose molecule, joined by a β-1,4-glycosidic bond. The monohydrate form signifies that for every lactose molecule, one molecule of water is incorporated within its crystalline structure. This water of crystallization enhances its stability in various industrial processes. Lactose monohydrate is widely used across different industries due to its physicochemical properties, including its slight sweetness, inertness, and compatibility with a wide range of active substances.
Chemical Composition and Structure
Lactose monohydrate (C₁₂H₂₂O₁₁·H₂O) is composed of two monosaccharides: D-glucose and D-galactose. The glycosidic bond between the two monosaccharides is a β-linkage, meaning the anomeric carbon of glucose (C1) is linked to the hydroxyl group of galactose (C4) in a beta configuration. The presence of one molecule of water in its crystalline structure (monohydrate form) distinguishes it from the anhydrous form of lactose. This water molecule is part of the crystalline matrix, enhancing the compound’s stability and making it easier to handle in various processes.
Physical Properties
Lactose monohydrate is a white to off-white crystalline powder with a slightly sweet taste. Its sweetness is about 20% that of sucrose, making it a mild sweetener. The compound is odorless and has a specific gravity of approximately 1.525 g/cm³. Lactose monohydrate has a melting point of 201–202°C, and upon heating, it may lose its water of hydration. It is moderately soluble in water, with solubility increasing with temperature. At room temperature, approximately 1.5 g of lactose dissolves in 10 mL of water. It is insoluble in most organic solvents like ethanol, making it useful for processes where low solubility in alcohol is required. The crystallinity of lactose monohydrate gives it good flow properties, making it suitable for applications that require high consistency in formulation and manufacturing.
Production Process
Lactose monohydrate is primarily derived from whey, a byproduct of cheese production. The production process typically follows several key steps:
Whey Collection and Filtration: After cheese production, whey is collected and filtered to remove fats and proteins (typically via ultrafiltration or microfiltration), leaving a liquid rich in lactose and other water-soluble components.
Concentration: The filtered whey undergoes concentration via vacuum evaporation, reducing the water content and increasing the concentration of lactose in solution. This step also helps reduce microbial load.
Crystallization: Lactose crystallization is initiated by cooling the concentrated solution under controlled conditions. The cooling induces lactose molecules to organize into a crystalline structure, forming lactose monohydrate crystals. The process is carefully managed to produce high-purity crystals with the desired particle size.
Separation and Drying: The crystals are separated from the remaining liquid via centrifugation or filtration, followed by washing to remove impurities. The wet crystals are then dried, typically in a fluidized bed dryer, to remove excess moisture while retaining the water of crystallization required for the monohydrate form.
Milling and Sieving: After drying, lactose monohydrate crystals are often milled to achieve the desired particle size distribution, which is crucial for applications in pharmaceuticals and other industries.
Applications
Medical: In the pharmaceutical industry, lactose monohydrate is extensively used as a diluent, filler, or carrier in the formulation of tablets and capsules. Its compressibility makes it ideal for direct compression tablet manufacturing. In dry powder inhalation (DPI) products, lactose monohydrate serves as a carrier to improve the dispersion and delivery of active pharmaceutical ingredients (APIs) to the lungs. Due to its inert nature and compatibility with a wide range of drugs, it is a preferred excipient for many formulations.
Cosmetics: Lactose monohydrate acts as a humectant and exfoliant in cosmetic formulations. It attracts moisture to the skin, promoting hydration, while its crystalline structure allows for gentle exfoliation in products like facial scrubs. Its mild nature makes it suitable for sensitive skin care products.
Food: In the food industry, lactose monohydrate is commonly used in infant formula to mimic the lactose content in human milk. It also acts as a bulking agent in baked goods, confectionery, and other processed foods. Its mild sweetness and stability during heat processing make it a versatile ingredient in food production. Additionally, lactose is used in the fermentation industry, particularly in dairy product fermentation.
Industrial and Biotechnological Uses: Lactose monohydrate is used as a carbon source in biotechnological processes, such as in the production of lactic acid, ethanol, and other biochemicals. Its controlled degradation by specific enzymes is a key process in dairy and fermentation industries.
Environmental and Safety Considerations
Lactose monohydrate is considered non-toxic and safe for human consumption and topical application. However, individuals with lactose intolerance—due to a deficiency of the enzyme lactase—may experience gastrointestinal discomfort when ingesting lactose-containing products. While lactose monohydrate is not considered an allergen, products containing it should be labeled appropriately to inform consumers with lactose sensitivity.
From an environmental perspective, lactose monohydrate is biodegradable and poses no significant environmental hazards. The production of lactose from whey, which is a byproduct of cheese production, is an environmentally friendly process that contributes to reducing waste in the dairy industry. The primary environmental consideration is the proper disposal of waste materials generated during the production process, such as the liquid whey permeate, which can be treated and repurposed.
In pharmaceutical and food industries, lactose monohydrate is regulated by various global standards, including Good Manufacturing Practices (GMP), ensuring its safety and purity in all applications. It is important to source lactose from suppliers that adhere to sustainability and safety standards to ensure product quality and minimize environmental impact.
Industrially it occurs in the form of a white crystalline powder or odourless white crystals containing galactose and glucose and, in the simple form of lactose, is found in a proportion of 2.5% to 3% in milk and is an isomer of sucrose.

What it is used for and where
Medicina e Farmaceutica
Artificial sweetener
It is commonly used as a binding excipient, filler binder and compression aid in pharmaceutical mixtures (1).
It is used in oral administration drugs as a spray-dried carrier (2).
Other uses
Biochemical research, adsorption chromatography.
For more information:
Typical commercial product characteristics Alpha-D-Lactose
| Appearance | White crystalline powder or granular |
| Boiling Point | 667.9ºC at 760 mmHg |
| Melting Point | 219ºC |
| Flash Point | 357.8ºC |
| Specific rotation | + 52 to + 52.8 degrees |
| Acidity | ≤ 0.2 (in H +) / (mmol / 100g) |
| Water insoluble matter | ≤ 0.005% |
| Heavy metal | ≤ 0.0005% |
| Density | 1.53 g/cm3 |
| Lactose | 99.56% |
| Ash | 0.09% |
| pH | 5.55 |
| Iron | ≤ 0.001% |
| Fat | ≤ 0.01% |
| Protein | 0.14% |
| Scorched particle | 7.50/mg |
| Coliform | <10 cfu/g |
| Bacillus cereus | <10 cfu/g |
| E coli | <10 cfu/g |
| Staph | <10 cfu/g |
| Mold | <10 cfu/g |
| Yeast | <10 cfu/g |
| SPC | <250 cfu/g |
| Listeria | Neg/25g |
| Salmonella | Neg/750g |
| PSA | 198.76000 |
| LogP | 1.652 |
 |  |
 |  |
Price 500mg €395
Synonyms:
References_________________________________________
(1) Mukherjee R, Halder A, Sansare S, Naik S, Chaudhuri B. A Simplex Centroid Design to Quantify Triboelectric Charging in Pharmaceutical Mixtures [published online ahead of print, 2020 Feb 24]. J Pharm Sci. 2020;S0022-3549(20)30074-5. doi:10.1016/j.xphs.2020.02.001
(2) Parvataneni DM, Devraj R, Mangamoori LN. Micelles entrapped microparticles technology: a novel approach to resolve dissolution and bioavailability problems of poorly water soluble drugs. J Microencapsul. 2020;37(3):254–269. doi:10.1080/02652048.2020.1729883
Abstract. Aim: Aim of this study was to design a solid oral delivery system for a weakly basic drug such as dasatinib (DAS), so as to achieve pH-independent dissolution and improved oral bioavailability.Methods: DAS was solubilised using sodium lauryl sulphate as an aqueous micellar system and such a system containing lactose monohydrate as carrier was spray-dried to obtain a solid mass. Subsequently, the DAS-solid was converted into a tablet using conventional tableting methods.Results: The dissolution study revealed pH-independent dissolution over a wide range of pH conditions. An in vivo bioavailability testing on rats revealed an improved Cmax and AUC0-24. Similarly, viability assay showed a better inhibitory effect of spray-dried dasatinib over the DAS.Conclusions: Micellar solubilisation and spray-drying technology can be approached to resolve poor dissolution and bioavailability of drugs belonging to biopharmaceutical classification system II and III. This technology is amenable to scale-up and has commercial potential.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2020-04-19 10:12:33 | Chemical Risk: |