Sodium levulinate is a chemical compound obtained from levulinic acid and is its salt.
The name describes the structure of the molecule:
- Sodium indicates the presence of sodium ions in the molecule.
- Levulinate is derived from the word "levulinic." and indicates that the molecule is a salt or ester of levulinic acid, a carboxylic acid derived from cellulose degradation.
Description of the raw materials used in its production:
- Levulinic Acid - Levulinic acid is a carboxylic acid that can be derived from renewable resources such as biomass. It can be obtained through the acid-catalyzed hydrolysis of cellulose or other carbohydrate sources.
- Sodium Hydroxide - Sodium hydroxide (NaOH) is an inorganic compound commonly used in the production of sodium salts. It is a strong base that reacts with levulinic acid to form sodium levulinate.
Industrial chemical synthesis step-by-step:
- Levulinic Acid Preparation - Levulinic acid can be prepared through various processes, such as the acid hydrolysis of biomass or the oxidation of glucose or fructose. These methods involve specific reaction conditions and catalysts to convert the starting materials into levulinic acid.
- Sodium Levulinate Formation - Levulinic acid is neutralized with sodium hydroxide in a controlled reaction. Sodium hydroxide reacts with the carboxylic acid group of levulinic acid, resulting in the formation of sodium levulinate and water.
- Purification - The crude sodium levulinate solution obtained from the reaction is typically purified to remove impurities and by-products. This purification process may involve filtration, crystallization, or other separation techniques.
- Drying and Packaging - The purified sodium levulinate is often dried to remove excess moisture and then packaged for distribution and use in various applications.
It occurs as a fine white powder.

What it is used for and where
Cosmetics
Skin conditioning agent - Miscellaneous. This ingredient has the task of modifying and improving the condition of the skin when it is damaged or dry, reducing flaking and restoring its elasticity.
It is used in cosmetic formulations also as a preservative and skin conditioning agent.
Food
Inserito prevalentemente in carni come antimicrobico.
Studies
This study investigated the use of sodium levulinate to prevent the growth of Listeria monocytogenes in chilled ready-to-eat meat products and showed that sodium levulinate is effective in inhibiting the growth of Listeria monocytogenes without affecting the taste of the meat (1).
Typical optimal commercial product characteristics Sodium levulinate
| Appearance | Fine white powder |
Boiling Point
| 242.9ºC at 760mmHg |
Density
| 1.129g/cm3 |
Flash Point
| 115ºC |
| PSA | 57.20000 |
Heavy Metals
| <0.002% |
| Water | 1% max |
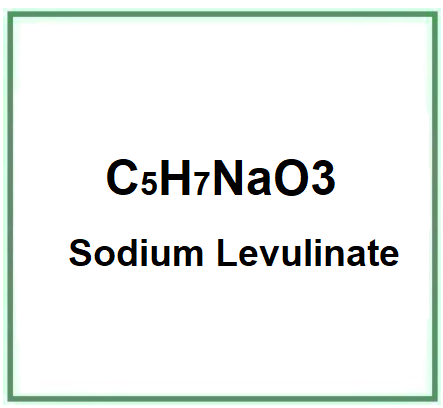
- Molecular Formula C5H7NaO3
- Molecular Weight 138.1 g/mol
- Exact Mass 138.02900
- CAS 19856-23-6
- UNII VK44E1MQU8
- EC Number:243-378-4
- DSSTox Substance ID: DTXSID60173608
- IUPAC sodium;4-oxopentanoate
- InChI=1S/C5H8O3.Na/c1-4(6)2-3-5(7)8;/h2-3H2,1H3,(H,7,8);/q;+1/p-1
- InChl Key RDKYCKDVIYTSAJ-UHFFFAOYSA-M
- SMILES CC(=O)CCC(=O)[O-].[Na+]
- MDL number
- PubChem Substance ID
- ChEBI
- ICSC
- NSC
- RTECS
- UN
- NCI
Synonyms:
- Levulinic acid sodium salt
- Sodium 4-oxovalerate
- sodium 4-oxopentanoate
- sodium 4-oxidanylidenepentanoate
- Pentanoic acid, 4-oxo-, sodium salt
References_______________________________________________________________________
(1) Thompson RL, Carpenter CE, Martini S, Broadbent JR. Control of Listeria monocytogenes in ready-to-eat meats containing sodium levulinate, sodium lactate, or a combination of sodium lactate and sodium diacetate. J Food Sci. 2008 Jun;73(5):M239-44. doi: 10.1111/j.1750-3841.2008.00786.x. Erratum in: J Food Sci. 2008 Aug;73(6):vii.
![]() Sodium levulinate
Sodium levulinate