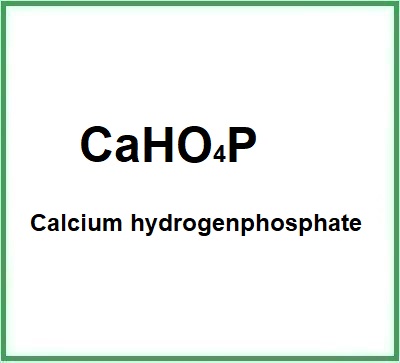
![]() Calcium hydrogenphosphate
Calcium hydrogenphosphate
Rating : 6.5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
8 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Calcium-hydrogenphosphate studies" about Calcium hydrogenphosphate Review Consensus 8 by Al222 (20707 pt) | 2021-Apr-11 10:15 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.

Vavrusova M, Danielsen BP, Garcia AC, Skibsted LH. Codissolution of calcium hydrogenphosphate and sodium hydrogencitrate in water. Spontaneous supersaturation of calcium citrate increasing calcium bioavailability. J Food Drug Anal. 2018 Jan;26(1):330-336. doi: 10.1016/j.jfda.2017.05.003.
Rehder S, Christensen NP, Rantanen J, Rades T, Leopold CS. High-shear granulation as a manufacturing method for cocrystal granules. Eur J Pharm Biopharm. 2013 Nov;85(3 Pt B):1019-30. doi: 10.1016/j.ejpb.2013.04.022. Epub 2013 May 16.
El Briak H, Durand D, Boudeville P. Study of a hydraulic DCPA/CaO-based cement for dental applications. J Mater Sci Mater Med. 2008 Feb;19(2):737-44. doi: 10.1007/s10856-007-3198-z.
Valentini Ganzerli MT, Maggi L, Crespi Caramella V, Berzero A. Preliminary results on the immobilisation of radionuclides from waters with specific adsorbers based on phosphate salts. Ann Chim. 2004 Nov;94(11):817-28. doi: 10.1002/adic.200490102.
Dong GC, Sun JS, Yao CH, Jiang GJ, Huang CW, Lin FH. A study on grafting and characterization of HMDI-modified calcium hydrogenphosphate. Biomaterials. 2001 Dec;22(23):3179-89. doi: 10.1016/s0142-9612(01)00070-9.
Dong GC, Lin FH, Yao CH, Jiang GJ, Huang CW. Preparation and characterization of surface-modified calcium hydrogenphosphate by hexamethylene diisocyanates. Biomed Sci Instrum. 2000;36:105-10.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Calcium hydrogenphosphate Review Consensus 8 by Al222 (20707 pt) | 2023-Oct-25 15:22 |
| Read the full Tiiip | (Send your comment) |
Calcium hydrogen phosphate is a chemical compound.
The name describes the structure of the molecule:
- Calcium indicates the presence of calcium ions in the molecule. Calcium is an alkaline earth metal and plays a crucial role in many biological functions, such as building bones and teeth.
- hydrogen indicates the presence of hydrogen ions in the molecule. Hydrogen is the lightest and most abundant element in the universe.
- phosphate indicates the presence of phosphate groups in the molecule. Phosphates are salts or esters of phosphoric acid and are key components of many biological molecules, like ATP and DNA.
Raw Materials Used in Production:
Calcium hydrogen phosphate can be obtained by treating phosphoric acid with calcium solutions.
Step-by-step Summary of Industrial Production Process.
- Preparation of raw materials. Phosphoric acid and calcium solutions are prepared.
- Neutralization. Phosphoric acid is reacted with a calcium solution (e.g., calcium hydroxide) to produce calcium hydrogen phosphate.
- Precipitation. The product precipitates out of the solution.
- Filtration and washing. The precipitate is filtered and washed to remove impurities.
- Drying. The product is dried to remove excess water.
- Grinding. The dry product is ground to the desired size.
Form and Color.
It appears as a white or almost white powder.

What it is for and where
Food Industry. Calcium hydrogen phosphate, also known as dibasic calcium phosphate, is used as a leavening agent in baked goods. It helps produce gas when mixed with acidifying agents, aiding the dough to rise.
Dietary Supplements. Being a source of calcium and phosphorus, it's often found in dietary supplements aimed at supporting bone health.
Pharmaceutical Industry. Used as an excipient in tablets and capsules, contributing to the compactness and cohesion of the final product.
Oral Hygiene. It can be used in some toothpastes as a mild abrasive to assist in plaque removal.

It is also used in medicine in various pharmaceutical applications: buffering agent; bulking agent; surface enhancer; solidifying agent; antioxidant synergist; stabiliser; emulsifier.
Studies
Calcium-hydrogenphosphate was considered as one of the main factors governing renal calculus formation. (1)
The mean area of remineralization (deltaZd-deltaZr) and mean percent remineralization (%R) in those chewing xylitol gum containing funoran and calcium hydrogenphosphate were significantly higher than the corresponding values for xylitol gum, sugar gum and gum base. Chewing xylitol gum containing funoran and calcium hydrogenphosphate has a significant effect on the remineralization of initial caries-like lesions of the teeth. (2)
To accelerate the healing of bone defects or for healing to take place, it is often necessary to fill them with suitable substance. Various artificial materials defects have been developed. Among these, calcium phosphates and bioactive glass have been proven to be biocompatibile and bioactive materials that can chemically bond with bone, and have been successfully used clinically for repair of bone defects and augmentation of osseous tissue. (3)
Calcium hydrogen phosphate studies
 |  |
Molecular Formula: CaHO4P CaHPO4
Molecular Weight: 136.06 g/mol
CAS: 7757-93-9
UNII TC2D6JAD40
UNII L11K75P92J
EC Number: 231-826-1
DSSTox Substance ID: DTXSID20872529
Synonims:
- Calcium phosphate, dibasic
- Calcium phosphate dibasic
Dicalcium phosphate
Phosphoric acid, calcium salt (1:1)
- Dicalcium Phosphate Anhydrous
- Dibasic calcium phosphate
- Monocalcium acid phosphate
References_________________________________________________________________________
(1) Reusz G, Szabó A. Hypercalciuria and postglomerular hematuria in children. The effects of thiazide on calcium excretion, urine saturation with respect to calcium-hydrogenphosphate and hematuria. Acta Paediatr Hung. 1990;30(1):63-71.
Abstract. Calcium-hydrogenphosphate was considered as one of the main factors governing renal calculus formation. The degree of saturation (expressed as activity product = AP) with respect to this phase was therefore calculated in urines of 36 hypercalciuric children (20 absorptive, 16 renal subtype) with isolated hematuria and 30 healthy controls. The effect of thiazide treatment on the urine saturation and on the evolution of hematuria was also investigated. The results were compared to the urinary calcium excretion (expressed as Ca/cr ratio). Urines of both hypercalciuric groups were saturated on basal conditions (AP above 3.5 x 10-6 mol2/l2; -lgAP below 6.4), the values differed significantly from those of the controls (-lgAP = 6.78 +/- 0.4 in the control-; 6.1 +/- 0.25 in absorptive-, 6.03 +/- 0.34 in renal hypercalciuria; p less than 0.001). Thiazide normalized the activity product in all groups. During thiazide therapy significant decrease in the occurrence of hematuria was noted (p less than 0.001 in both hypercalciuric groups). These data furnish further evidence on the relation of hypercalciuria and postglomerular hematuria. Simultaneous determinations of the state of saturation may provide further information on the "stone forming potential" of the urines investigated.
(2) Thaweboon S, Nakornchai S, Miyake Y, Yanagisawa T, Thaweboon B, Soo-Ampon S, Lexomboon D. Remineralization of enamel subsurface lesions by xylitol chewing gum containing funoran and calcium hydrogenphosphate. Southeast Asian J Trop Med Public Health. 2009 Mar;40(2):345-53.
Abstract. Casein phosphopeptide-amorphous calcium phosphate nanocomplexes (CPP-ACP) exhibit anticariogenic potential in laboratory, animal, and human in situ experiments. The aim of this study was to determine the ability of CPP-ACP in sugar-free chewing gum to remineralize enamel subsurface lesions in a human in situ model. Thirty subjects in randomized, cross-over, double-blind studies wore removable palatal appliances with six human-enamel half-slabs inset containing sub-surface demineralized lesions. The appliances were inserted immediately before gum-chewing for 20 min and then retained for another 20 min. This was performed four times per day for 14 days. At the completion of each treatment, the enamel half-slabs were paired with their respective demineralized control half-slabs, embedded, sectioned, and subjected to microradiography and densitometric image analysis, for measurement of the level of remineralization. The addition of CPP-ACP to either sorbitol- or xylitol-based gum resulted in a dose-related increase in enamel remineralization, with 0.19, 10.0, 18.8, and 56.4 mg of CPP-ACP producing an increase in enamel remineralization of 9, 63, 102, and 152%, respectively, relative to the control gum, independent of gum weight or type.
(3) Lin FH, Dong GC, Chen KS, Jiang GJ, Huang CW, Sun JS. Immobilization of Chinese herbal medicine onto the surface-modified calcium hydrogenphosphate. Biomaterials. 2003 Jun;24(13):2413-22. doi: 10.1016/s0142-9612(03)00031-0.
Abstract. To accelerate the healing of bone defects or for healing to take place, it is often necessary to fill them with suitable substance. Various artificial materials defects have been developed. Among these, calcium phosphates and bioactive glass have been proven to be biocompatibile and bioactive materials that can chemically bond with bone, and have been successfully used clinically for repair of bone defects and augmentation of osseous tissue. However, those bioceramics have only the property of osteoconduction without any osteoinduction. Many ligands have been physicochemically absorbed onto substrates to enhance cell-substrate interactions. Although widely developed, they are still limited to use in long-term implantation because of their half-life period. Thus, some interfacial modification will be required for enhancing the efficacy of the delivery system. These models involve the immobilization of biologically active ligands of natural and synthetic origin onto various substrates to produce an interface with stronger chemical bond between ligand and substrate. The advantage of covalently immobilizing a ligand is that a chemical bond is present to prevent ligand or medicine from desorption. In our study, a two-step chemical immobilization was performed to surface-modified calcium hydrogenphosphate powders. The first was to modify the surface of calcium hydrogen-phosphate (CHP) with a coupling agent of hexanmethylene diisocyanate (HMDI). CHP surface modified by HMDI is abbreviated as MCHP. The linkage between CHP and HMDI will be characterized by FTIR. The second step was to immobilize chemically Gusuibu onto MCHP. Moreover, the sorption and desorption of Gusuibu was evaluated and quantitatively analyzed by spectrophotometer and HPLC. Bioceramic CHP was surface-modified by a two-step chemical immobilization. First, the surface of calcium hydrogen-phosphate (CHP) was successfully modified with coupling agent of hexanmethylene diisocyanate (HMDI). The first step was also activated the surface of CHP to induce primary amine terminator. The reaction of this functional group with Gusuibu was the second step. We confirmed simultaneously that Gusuibu could be immobilized chemically onto the surface of MCHP. Although some immobilized Gusuibu was also released rapidly at the first 12h, the degree of the released Gusuibu was lower than both by Gusuibu-adsorbing MCHP and Gusuibu-adsorbing CHP.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2021-04-11 09:32:56 | Chemical Risk: |