![]() Sodium saccharin
Sodium saccharin
Rating : 5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Avoid excessive amounts (1)10 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Saccharin sodium studies" about Sodium saccharin Review Consensus 10 by AColumn (9336 pt) | 2022-Jul-04 19:10 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Das Neves, A. O. C., Melo, T. A., Nunes, M. B., & Romano, C. C. (2020). Effect of saccharin sodium and the sodium cyclamate on human cells treated with lactobacillus plantarum lp62. Food & Nutrition Journal.
Abstract. The commercial sweeteners have been suggested for diabetics and people who need to lose weight. Its use has grown on a global scale, as well as health problems related to it. Despite their discovery over a hundred years, suspicion and uncertainty remain giving rise to numerous discussions on the safety of human health. Thus, this study aimed to evaluate the effect of saccharin and cyclamate sodic on the viability of human cells, as well as the possible protective effect of Lactobacillus plantarum LP62 on cells treated with the sweeteners. We found that human cells exposed to high concentrations of sweeteners reduced their viability, with cyclamate being more harmful than saccharin. The results show the efficacy of lactobacilli in reducing the production of inflammatory cytokines in human cells in the presence of a sweetener, especially in peripheral blood mononuclear cells. Thus, we indicate the LP62 as a promising probiotic candidate and emphasize the need for greater scientific investment in this area of knowledge in order to better clarify the effect of sweeteners on human cells and microorganisms and for understanding of the combination thereof.
Shen T, Li J. Drinking Non-nutritive Sweetness Solution of Sodium Saccharin or Rebaudioside a for Guinea Pigs: Influence on Histologic Change and Expression of Sweet Taste Receptors in Testis and Epididymis. Front Nutr. 2021 Aug 5;8:720889. doi: 10.3389/fnut.2021.720889.
Abstract. Saccharin sodium and rebaudioside A are extensively used as non-nutritive sweeteners (NNSs) in daily life. NNSs elicit a multitude of endocrine influences on animals, differing across species and chemically distinct sweeteners, whose exposure induce activation of sweet taste receptors in oral and extra-oral tissues with consequences of metabolic changes.
Saito J, Nadatani N, Setoguchi M, Nakao M, Kimura H, Sameshima M, Kobayashi K, Matsumoto H, Yoshikawa N, Yokoyama T, Takahashi H, Suenaga M, Watanabe R, Imai K, Obara M, Hashimoto M, Yamamoto K, Fujiwara N, Sakata W, Nagai H, Enokihara T, Katayama S, Takahashi Y, Araki M, Iino K, Akiyama N, Katsu H, Fushimi K, Takeda T, Torimoto M, Kishi R, Mitsuya N, Kihara R, Hasegawa Y, Hamada Y, Kimura T, Wada M, Tanzawa A, Yamatani A. Potentially harmful excipients in neonatal medications: a multicenter nationwide observational study in Japan. J Pharm Health Care Sci. 2021 Jul 1;7(1):23. doi: 10.1186/s40780-021-00208-9.
Abstract. Background: A multicenter investigation of neonate exposure to potentially harmful excipients (PHEs) in neonatal intensive care units (NICUs) in Japan has not been conducted. Methods: A multicenter nationwide observational study was conducted. Neonate patient demographic data and information on all medicines prescribed and administered during hospitalization on 1 day between November 2019 and March 2021 were extracted from the medical records. Nine PHEs, paraben, polysorbate 80, propylene glycol, benzoates, saccharin sodium, sorbitol, ethanol, benzalkonium chloride, and aspartame, were selected. PHEs were identified from the package insert and the Interview Form. The quantitative daily exposure was calculated if quantitative data were available for each product containing the PHE.
Williams, R. A. (1970). Human detectability thresholds for saccharin, sodium saccharin, and sodium chloride. Journal of Comparative and Physiological Psychology, 70(1p1), 113.
Abstract. The detectability threshold was defined as the concentration which was reported as "different" from water 50% of the time. The average thresholds, with little between-S variability, were: saccharin, 1.6 * 10-5 M; sodium saccharin, 2 * 10-5 M; and NaCl, 2.2 * 10-3 M.
Cooney, D. O. (1977). Saccharin sodium as a potential sweetener for antidotal charcoal. American Journal of Health-System Pharmacy, 34(12), 1342-1344.
Abstract. This study explored if saccharin sodium, as a sweetener in activated charcoal formulations, would be sufficiently available to provide sweetness while not severely reducing charcoal’s adsorption capacity.
Kumar Das, M., Hao, R., Srikaen, E., Thueploy, A., Limpanart, S., Boonyongmaneerat, Y., & Qin, J. Q. (2015). Effect of saccharin sodium on the microstructure and hardness of electrodeposited Ni-W coatings. In Key Engineering Materials (Vol. 659, pp. 535-539). Trans Tech Publications Ltd.
Abstract. Nickel-tungsten alloy were fabricated by electrodeposition on carbon steel. The influences of saccharin sodium on the coatings were analyzed using XRD, SEM, and hardness tester. This study reveals that the presence of saccharine sodium has a profound impact on the morphology of the coatings. Moreover, the addition of saccharine sodium tends to enhance the tungsten content in the coatings along with hardness and the grain size of the deposits.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Sodium saccharin Review Consensus 10 by Al222 (20702 pt) | 2023-Jun-17 16:37 |
| Read the full Tiiip | (Send your comment) |
Saccharin sodium is a chemical compound, the sodium salt of saccharin has a sweetening power of about 300-400 times that of sugar.
The process of synthesis from phthalic anhydride, also known as orthophthalic anhydride. takes place in different stages which, to simplify, are:
- Sulphonation. Phthalic anhydride is sulphonated with concentrated sulphuric acid to produce o-sulphobenzoic acid.
- Conversion. Sulphonic acid is then converted to its anhydride using acetic anhydride.
- Reaction. The sulphonate compound is heated with ammonia, leading to the substitution of a sulphonyl group with the amine. Sulphonamide is thus formed.
- Cyclization and oxidation. The sulphonamide compound undergoes a cyclization reaction to produce saccharin, which is then oxidised to give the final product, saccharin.
- Sodium salt formation. Saccharin is treated with sodium hydroxide (NaOH) to form the sodium salt, sodium saccharin.
It takes the form of a white crystalline powder with an intensely sweet, odourless flavour. Stable. Incompatible with strong oxidising agents. Soluble in acetone.

What it is used for and where
Food and Pharmaceuticals
It is a non-caloric artificial sweetener widely used as a sugar substitute to control body weight or blood sugar, absorbed through intact intestinal epithelial cells and is not metabolised.
Saccharin was discovered in 1879 by researchers at Johns Hopkins University and is low in calories. Its sweetening power is about 450 times stronger than that of sucrose.
Sodium saccharin, which is a salt of saccharin, is often found commercially in pharmaceuticals and cosmetics and is used like saccharin as it is, from a practical point of view, the same component albeit with a different formula and molecular weight.
It is soluble in water and does not create hepatotoxicity problems. It is labelled with the number E954 in the list of European additives as a sweetener.
However, high doses of sodium saccharin may prove dangerous to human health.
Studies
In recent decades, extensive discussions have been on the impact of artificial sweeteners on cancer risk. The present study aimed to evaluate the interaction of saccharin and sodium saccharinate with the human p53 gene promoter. This study may contribute to assessing the potential carcinogenicity risk of this sweetener and to the design and application of new and safer artificial sweeteners, and the other studies mentioned discuss the possible risks of saccharin intake in the human body (1).
As with other sweeteners, the field of scientific research is teeming with studies on its food safety and the results are still mixed. Hundreds of clinical studies have tested saccharin in laboratory rats, monkeys and humans. In practice, some studies claim that impurities in commercial saccharin may have been responsible for tumours (2), whereas according to the current literature, the possible risk of artificial sweeteners to induce cancer appears to be negligible (3). Even relatively high levels of saccharin intake among diabetic subjects have not been found to increase the risk of cancer in general (4).
Cosmetics
Flavoring agent. The purpose of this ingredient is to modify the solution to impart a certain flavour. Natural flavouring extracts are rather expensive, so the cosmetic and pharmaceutical industries resort to synthesised substances that have sensory characteristics mostly similar to natural flavourings or are naturally equivalent. This ingredient is isolated through chemical processes or is synthesised from chemicals. It is also referred to as Aroma.
Fragrance. It plays a decisive and important role in the formulation of cosmetic products as it provides the possibility of enhancing, masking or adding fragrance to the final product, increasing its marketability. It is able to create a perceptible pleasant odour, masking a bad smell. The consumer always expects to find a pleasant or distinctive scent in a cosmetic product.
Oral care agent. This ingredient can be placed in the oral cavity to improve and/or maintain oral hygiene and health, to prevent or improve a disorder of the teeth, gums, mucous membrane. It provides cosmetic effects to the oral cavity as a protector, cleanser, deodorant.
Typical commercial product characteristics Saccharin sodium
| Appearance | White powder |
| Boiling Point | 438.9ºC at 760 mmHg |
| Melting Point | >300°C |
| Flash Point | 219.3ºC |
| PSA | 77.9400 |
| LogP | 1.08240 |
| Vapor Pressure | 1.77E-08mmHg at 25°C |
| Water Solubility | >=10 g/100 mL at 20 ºC |
| Loss on Drying | ≤5.00% |
| Total Heavy Metals | ≤10ppm |
| Arsenic | ≤1ppm |
| Lead | ≤2ppm |
| Cadmium | ≤1ppm |
| Mercury | ≤0.1ppm |
| Total Plate | ≤5000cfu/g |
| Yeast & Mold | ≤100cfu/g |
| Storage | 0-6°C |
 |  |
 |  |
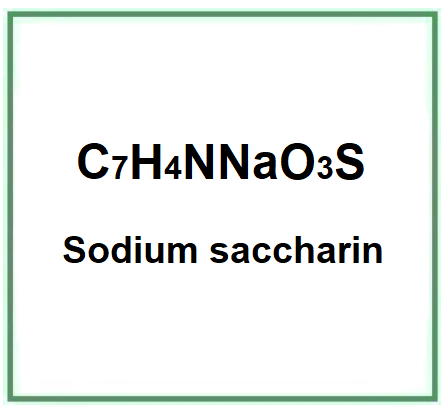
- Molecular Formula C7H4NNaO3S
- Molecular Weight 205.17 g/mol
- Exact Mass 204.980957
- CAS 128-44-9 6155-57-3
- UNII I4807BK602
- EC Number: 204-886-1 612-173-5
- DSSTox Substance ID: DTXSID5021253
- IUPAC sodium;1,1-dioxo-1,2-benzothiazol-2-id-3-one
- InChI=1S/C7H5NO3S.Na/c9-7-5-3-1-2-4-6(5)12(10,11)8-7;/h1-4H,(H,8,9);/q;+1/p-1
- InChl Key WINXNKPZLFISPD-UHFFFAOYSA-M
- SMILES C1=CC=C2C(=C1)C(=O)[N-]S2(=O)=O.[Na+]
- MDL number MFCD00149605
- PubChem Substance ID 24871502
- ChEBI 32112
- Beilstein 3599229
- FEMA 2997
- RXCUI 1362914
- RTECS DE4550000
Synonyms
- Sodium saccharine
- Sodium 3-oxo-3H-benzo[d]isothiazol-2-ide 1,1-dioxide
- Saccharin, sodium
- Sodium saccharinate
- Saccharin sodium anhydrous
- Cristallose
References_______________________________________________________________________
(1) Mansourian M, Mahnam K, Rajabi HR, Roushani M, Doustimotlagh AH. Exploring the binding mechanism of saccharin and sodium saccharin to promoter of human p53 gene by theoretical and experimental methods. J Biomol Struct Dyn. 2020 Feb;38(2):548-564. doi: 10.1080/07391102.2019.1582438.
Li J, Shen T, Shi F, Fu Y. Influences of non-nutritive sweeteners on ovarian and uterine expression of T1R2 and T1R3 in peripubertal female guinea pigs. Anim Sci J. 2020 Jan-Dec;91(1):e13348. doi: 10.1111/asj.13348.
Cuykx M, Beirnaert C, Rodrigues RM, Laukens K, Vanhaecke T, Covaci A. Exposure of HepaRG Cells to Sodium Saccharin Underpins the Importance of Including Non-Hepatotoxic Compounds When Investigating Toxicological Modes of Action Using Metabolomics. Metabolites. 2019 Nov 4;9(11):265. doi: 10.3390/metabo9110265.
Rehn S, Onuma T, Rooney KB, Boakes RA. Sodium saccharin can be more acceptable to rats than pure saccharin. Behav Processes. 2018 Dec;157:188-191. doi: 10.1016/j.beproc.2018.09.009.
Wang Y, Li C, Guo B, Jin D, Li X, Wang C. Theoretical risk assessment for dietary exposure to sodium saccharin among residents in Nanjing City. Wei Sheng Yan Jiu. 2020 Nov;49(6):1008-1013. Chinese. doi: 10.19813/j.cnki.weishengyanjiu.2020.06.023.
Qu J, Chen X, Zhou J, Li H, Mai W. Treatment of real sodium saccharin wastewater using multistage contact oxidation reactor and microbial community analysis. Bioresour Technol. 2019 Oct;289:121714. doi: 10.1016/j.biortech.2019.121714.
Ashby J. The genotoxicity of sodium saccharin and sodium chloride in relation to their cancer-promoting properties. Food Chem Toxicol. 1985 Apr-May;23(4-5):507-19. doi: 10.1016/0278-6915(85)90145-0.
Chappel CI. A review and biological risk assessment of sodium saccharin. Regul Toxicol Pharmacol. 1992 Jun;15(3):253-70. doi: 10.1016/0273-2300(92)90037-a.
Nakama KA, Dos Santos RB, Serpa P, Maciel TR, Haas SE. Organoleptic excipients used in pediatric antibiotics. Arch Pediatr. 2019 Oct;26(7):431-436. doi: 10.1016/j.arcped.2019.09.008.
EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López-Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Brantom P, Dusemund B, Van Beelen P, Westendorf J, Gregoretti L, Manini P, Chesson A. Safety and efficacy of sodium saccharin when used as a feed flavour for piglets, pigs for fattening, calves for rearing and calves for fattening. EFSA J. 2018 Mar 22;16(3):e05208. doi: 10.2903/j.efsa.2018.5208.
Koland M, Sandeep V, Charyulu N. Fast Dissolving Sublingual Films of Ondansetron Hydrochloride: Effect of Additives on in vitro Drug Release and Mucosal Permeation. J Young Pharm. 2010 Jul;2(3):216-22. doi: 10.4103/0975-1483.66790.
(2) Arnold DL. Two-generation saccharin bioassays. Environ Health Perspect. 1983 Apr;50:27-36. doi: 10.1289/ehp.835027.
(3) Weihrauch MR, Diehl V. Artificial sweeteners--do they bear a carcinogenic risk? Ann Oncol. 2004 Oct;15(10):1460-5. doi: 10.1093/annonc/mdh256.
Lohner S, Toews I, Meerpohl JJ. Health outcomes of non-nutritive sweeteners: analysis of the research landscape. Nutr J. 2017 Sep 8;16(1):55. doi: 10.1186/s12937-017-0278-x.
(4) Armstrong B, Lea AJ, Adelstein AM, Donovan JW, White GC, Ruttle S. Cancer mortality and saccharin consumption in diabetics. Br J Prev Soc Med. 1976 Sep;30(3):151-7. doi: 10.1136/jech.30.3.151.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-11-01 12:46:51 | Chemical Risk: |