Sodium Cocoate is a chemical compound, sodium salt of coconut fatty acid.
The name describes the structure of the molecule:
- Sodium indicates the presence of sodium ions in the molecule.
- Cocoate, derived from the word "coconut." indicates that the molecule is a salt or ester of the acid obtained from the saponification of coconut oils.
Description of the raw materials used in its production:
- Coconut oil is a vegetable oil extracted from the meat or kernel of mature coconuts. It is primarily composed of medium-chain fatty acids such as lauric acid, myristic acid, and palmitic acid.
- Sodium hydroxide, commonly known as caustic soda, is a highly alkaline chemical substance. It is used for saponification, which is the chemical process where a fat or oil is converted into soap.
Industrial chemical synthesis step-by-step:
- Saponification - Coconut oil is heated and mixed with a solution of sodium hydroxide (NaOH) to initiate the saponification reaction. During this reaction, the fatty acids present in the coconut oil combine with the sodium from sodium hydroxide to form sodium cocoate.
- Separation - After saponification, the mixture is allowed to cool and solidify. Subsequently, water and other impurities are separated from the sodium cocoate through separation processes such as centrifugation or filtration.
- Purification - The sodium cocoate obtained from separation is purified to remove any residual impurities. This can be done through refining processes such as crystallization or solvent treatment.
- Formulation - The purified sodium cocoate is used as a key ingredient in the production of soaps and detergents. It is often combined with other surfactants, moisturizing agents, or fragrances to achieve the desired properties in the final product.
It appears in the form of a white powder.

What it is for and where
Cosmetics
Cleansing agent. Ingredient that cleanses skin without exploiting the surface-active properties that produce a lowering of the surface tension of the stratum corneum.
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Commercial Applications
Soap Industry. Sodium Cocoate is the sodium salt of the fatty acids present in coconut oil. It is a primary ingredient in soap making.
Surfactant. Due to its cleansing properties, it's used as a foaming and cleansing agent in various cleaning products.
Skin Care Products. Found in cleansers and soaps due to its emollient properties, which help keep the skin soft and moisturized.
Hair Care Products. Can be found in some shampoos and conditioners where it helps in cleansing and conditioning the hair.
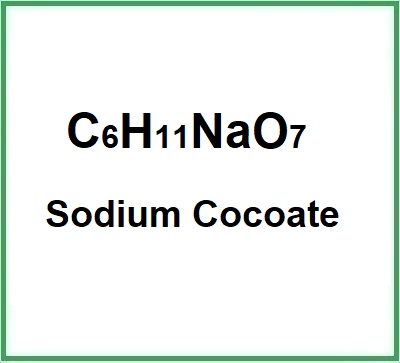
- Molecular Formula: C6H11NaO7
- CAS: 61789-31-9
- EC Number: 263-050-4
- UNII R1TQH25F4I
Synonyms:
- Coconut oil fatty acids, sodium salt
- Sodium coconate
- Soap, coconut oil
![]() Sodium Cocoate
Sodium Cocoate