Cetearyl Ethylhexanoate (Cetearyl Octanoate) is an ester used primarily in cosmetic products as an emollient. It enhances the texture of formulations and provides a silky, smooth feel on the skin, making it a popular ingredient in creams, lotions, and hair care products. Cetearyl ethylhexanoate helps reduce the greasiness often associated with oil-based products, leaving the skin soft and smooth without an oily residue. It is suitable for all skin types, including sensitive skin, and is known for its fast absorption and ability to improve the distribution of other active ingredients in formulations.
Chemical Composition and Structure
Cetearyl ethylhexanoate is an ester formed by combining cetearyl alcohol (a mixture of cetyl and stearyl alcohols) and ethylhexanoic acid. Its chemical structure features a long chain, which gives it emollient properties, making it ideal for softening and moisturizing the skin and hair. This combination of fatty alcohols and organic acids enhances its ability to provide a smooth, lightweight feel to formulations.
Physical Properties
Cetearyl ethylhexanoate is a clear, colorless, and odorless liquid with a lightweight texture or a white powder. It is insoluble in water but mixes easily with oils and other lipophilic ingredients. Due to its rapid absorption, it leaves a silky, non-greasy finish, making it suitable for lightweight formulations like fast-absorbing serums and lotions.

The name describes the structure of the molecule.
- "Cetearyl" refers to the mixture of cetyl and stearyl alcohols, fatty alcohols.
- "Ethylhexanoate" is the ester formed by ethyl alcohol and hexanoic acid.
Description of the raw materials used in its production:
- Cetearyl alcohol is a compound derived from the combination of cetyl alcohol and stearyl alcohol. It is a solid fatty substance at room temperature and is often used as an emulsifier and stabilizer in cosmetic products.
- Ethylhexanoic acid is a fatty acid derived from plant or animal sources. It is also known as caprylic acid and is commonly used as an emollient and conditioning agent in the production of cosmetic products.
The synthesis process takes place in several stages:
Step 1: Preparation of raw materials: stearilic alcohol, ethyl alcohol and hexanoic acid.
Step 2: Esterification. Fatty alcohols (cetyl and stearyl alcohols) are reacted with ethylhexaanoic acid in the presence of an acid catalyst to form the ester, Cetearyl Ethylhexanoate.
Step 3: Purification. The resulting Cetearyl Ethylhexanoate is purified to remove any raw materials and unreacted by-products. This is typically done through a series of washing and filtration steps.
Step 4: Drying. Purified Cetearyl Ethylhexanoate is then dried to remove any residual solvent.
What it is used for and where
Cosmetics
This chemical compound is used by the cosmetic industry as an emollient, emulsifier and thickening agent.
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Hair conditioning agent. A significant number of ingredients with specific and targeted purposes may co-exist in hair shampoo formulations: cleansers, conditioners, thickeners, matting agents, sequestering agents, fragrances, preservatives, special additives. However, the indispensable ingredients are the cleansers and conditioners as they are necessary and sufficient for hair cleansing and manageability. The others act as commercial and non-essential auxiliaries such as: appearance, fragrance, colouring, etc. Hair conditioning agents have the task of increasing shine, manageability and volume, and reducing static electricity, especially after treatments such as colouring, ironing, waving, drying and brushing. They are, in practice, dispersants that may contain cationic surfactants, thickeners, emollients, polymers. The typology of hair conditioning agents includes: intensive conditioners, instant conditioners, thickening conditioners, drying conditioners. They can perform their task generally accompanied by other different ingredients.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
It is also used as dispersing agent, base oil agent and lip gloss agent in lipstick, oil agent as softener in lotions and creams, as emollient in personal skin care products. Amounts normally included in formula: 1-5% in skin care products, 0.2-1.0% in shampoo and 1-4% in hair care products
Safety
Cetearyl Ethylhexanoate Is generally considered safe (1).
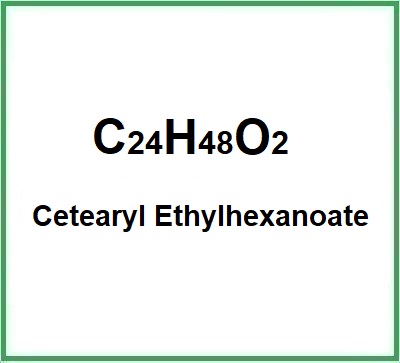
- Molecular Formula: C24H48O2
- PMolecular Weight: 368.6
- CAS: 90411-68-0 59130-69-7
- UNII 9M64UO4C25
- EC Number: 291-445-1
Synonyms:
- Hexadecyl 2-ethylhexanoate
- Cetyl 2-ethylhexanoate
- Perceline oil
- Exceparl HO
- SCHEMBL15239
- Pelemol 168
- Schercemol CO
References_____________________________________________________________________
(1) Belsito, M. D., Hill, R. A., Klaassen, C. D., Liebler, D. C., Marks Jr, J. G., & Ronald, C. Amended Safety Assessment of Alkyl Ethylhexanoates as Used in Cosmetics.
Kiselmann, C., Dobler, D., Schmidts, T., Eicher, A. C., Möbs, C., Pfützner, W., & Runkel, F. (2018). Development of a skin-friendly microemulsion for dermal allergen-specific immunotherapy. International Journal of Pharmaceutics, 550(1-2), 463-469.
Abstract. Due to their role in immune responses, the skin dendritic cells (i.e. epidermal Langerhans cells and dermal dendritic cells) have become of great interest to researchers in the past decades. A dermal administration of allergens could target these professional antigen-presenting cells directly and build up immunotolerance. Additionally, many of the adverse side effects, which are seen in the current state of the art specific immunotherapy routes, could be avoided. Therefore, in this study a penetration enhancing microemulsion was developed and its physicochemical properties were determined under several storage conditions. The influence of different preservatives on the microemulsion stability was observed. We examined epidermal penetration of Alexa Fluor-647 labelled bee-venom phospholipase A2 (Api m 1) using the Franz diffusion cell set up and confocal laser-scanning microscopy. First results of an in-vivo Api m 1-allergic mouse model indicated the protective efficacy of dermal AIT with our newly developed microemulsion. Summarily, the developed microemulsion is a suitable, stable drug delivery system for the topical administration of proteogenic allergens into the epidermis and is able to reach dendritic cells in the skin.
![]() Cetearyl Ethylhexanoate
Cetearyl Ethylhexanoate