Calcium stearate is a chemical compound formed by the reaction of stearic acid with calcium oxide, insoluble in water and some solvents,
Calcium stearate, the calcium salt of stearic acid, commonly used in various cosmetic and industrial applications. It is valued for its multifunctional properties, including its role as an anticaking agent, colorant, emulsion stabilizer, and viscosity controller.
Chemical Composition and Structure
Calcium stearate is an organic compound with the chemical formula Ca(C18H35O2)2. It consists of calcium ions combined with stearic acid, a long-chain fatty acid. This combination gives the compound its unique properties and wide range of applications.
Calcium stearate typically appears as a fine white powder. It is insoluble in water but soluble in oils and organic solvents. The compound is known for its stability, resistance to heat, and ability to form gels with oils, making it highly useful in various formulations.
Chemical Industrial Synthesis Process
- Preparation of reagents. The main raw materials include stearic acid and a calcium compound such as calcium chloride (CaCl₂) or calcium hydroxide (Ca(OH)₂).
- Saponification. The stearic acid is heated and treated with a sodium hydroxide (NaOH) solution to form sodium stearate (sodium stearate soap).
- Precipitation of calcium stearate. The calcium chloride or calcium hydroxide is slowly added to the sodium stearate solution with constant stirring. This reaction forms a precipitate of calcium stearate.
- Filtration. The resulting suspension is filtered to separate the solid calcium stearate precipitate from the aqueous solution.
- Washing. The calcium stearate precipitate is washed with deionized water to remove any soluble impurities and residual reagents.
- Drying. The washed calcium stearate is dried at controlled temperatures to remove residual moisture and obtain a dry powder.
- Grinding. The dried calcium stearate is ground to obtain a fine and uniform powder. This step may involve the use of ball mills or other grinding machinery.
- Classification. The dried powder is classified to ensure a uniform particle size. This step may involve sieving or the use of air classifiers.
- Stabilization. The calcium stearate powder is stabilized to ensure its stability during transportation and storage, preventing aggregation and degradation.
- Quality control. The calcium stearate undergoes rigorous quality testing to ensure it meets standards for purity, safety, and functionality. These tests include chemical analysis, spectroscopy, and physical tests to determine particle size and rheological properties.
It appears in the form of a white powder

Industrial applications
Plastic. Calcium stearate id widely used by the industry with nano-composite technology as a thickener, lubricant, non-toxic molding stabilizer for PVC. It is regarded as a classical pro-oxidant agent for polyolefin (a co-extruded multilayer film that is composed of polyethylene and polypropylene).
In rubber processing as a plasticizer, it has the property of making rubber more natural synthetic, soft with minimal effects on polymerization.
In the textile industry it is a waterproofing agent for fabrics, textiles and in the paint industry it makes the mixture smoother.
It has countless other applications, from plasticizer to the production of the core of pencils, polyolefin fiber, waterproof materials, paper, etc..
In the food industry it is used as a caking agent and classified at GRAS, 0.50%, 0.75%, and 1.00% wt/wt.
In medicinal tablets, Calcium stearate functions as a lubricant because pharmaceutical tablets are produced through a series of batch steps that end with compression into a mold using a tablet press. Lubricants reduce friction between the tablet and the metal surface of the mold to ensure that the tablet is ejected properly from the press (1).
Anti-caking agents are often added to powder systems to delay or prevent agglomeration, and in this study on the rate of vitamin C degradation, several anti-caking agents including calcium stearate were controlled (2).
In medicine, drug delivery from pellets (neutral microgranules) faces various issues such as robustness, risk of ethanol-induced dose dumping. hydrophobic calcium stearate can be considered a suitable matrix system that minimizes the risk of ethanol-induced dose dumping for some active pharmaceutical ingredients (3).
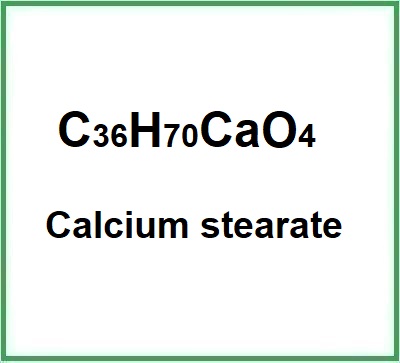
Molecular Formula: C36H70CaO4 C36H70O4.Ca
Molecular Weight: 607.0
Linear Formula: (CH3(CH2)16-COO)2Ca
CAS: 1592-23-0 66071-81-6 8000-75-7
UNII 776XM7047L
EC Number: 216-472-8 216-472-8 616-781-1
DSSTox Substance ID: DTXSID7027419 DTXSID5029392
MDL number MFCD00036390
PubChem Substance ID 57648169
Bellstein: 3919698
NACRES: na.21
Synonyms:
- Calcium distearate
- Calcium octadecanoate
References______________________________________________________________________
(1) Morin G, Briens L. The effect of lubricants on powder flowability for pharmaceutical application. AAPS PharmSciTech. 2013 Sep;14(3):1158-68. doi: 10.1208/s12249-013-0007-5.
Abstract. Pharmaceutical tablets are manufactured through a series of batch steps finishing with compression into a form using a tablet press. Lubricants are added to the powder mixture prior to the tabletting step to ensure that the tablet is ejected properly from the press. The addition of lubricants also affects tablet properties and can affect the behavior of the powder mixture. The objective of this research was to investigate the effect of lubricants on powder flowability as flowability into the tablet press is critical. Four lubricants (magnesium stearate, magnesium silicate, stearic acid, and calcium stearate) were mixed, in varying amounts, with spray-dried lactose. In addition, magnesium stearate was also mixed with placebo granules from a high-shear granulator. Measurements based on avalanche behavior indicated flowability potential and dynamic density and were more sensitive to changes in the mixture and provided a more accurate and reproducible indication of flowability than traditional static measurements. Of the tested lubricants, magnesium stearate provided the best increase in flowability even in the low amounts commonly added in formulations.
(2) Lipasek RA, Taylor LS, Mauer LJ. Effects of anticaking agents and relative humidity on the physical and chemical stability of powdered vitamin C. J Food Sci. 2011 Sep;76(7):C1062-74. doi: 10.1111/j.1750-3841.2011.02333.x.
(3) Jedinger N, Schrank S, Mohr S, Feichtinger A, Khinast J, Roblegg E. Alcohol dose dumping: The influence of ethanol on hot-melt extruded pellets comprising solid lipids. Eur J Pharm Biopharm. 2015 May;92:83-95. doi: 10.1016/j.ejpb.2015.02.022.
![]() Calcium stearate
Calcium stearate