![]() Crospovidone
Crospovidone
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
18 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Crospovidone studies" about Crospovidone Review Consensus 10 by Al222 (20707 pt) | 2022-Nov-21 18:57 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Jain SP, Mehta DC, Shah SP, Singh PP, Amin PD. Melt-in-mouth pellets of fexofenadine hydrochloride using crospovidone as an extrusion-spheronisation aid. AAPS PharmSciTech. 2010 Jun;11(2):917-23. doi: 10.1208/s12249-010-9443-7.
Abstract. Microcrystalline cellulose (MCC) is well established as an extrusion spheronisation aid for the preparation of pellets. Crospovidone (Polyplasdone XL-10) is compared with microcrystalline cellulose for the preparation of melt-in-mouth pellets. Taste-masked fexofenadine hydrochloride was incorporated in the melt-in-mouth formulation. Crospovidone was found to be well suited as extrusion-spheronisation aid for the preparation of melt-in-mouth pellets. The great advantage of crospovidone is, however, the disintegrating properties of the pellets after only a short time of exposure to liquid. Crospovidone was successfully employed as an extrusion-spheronisation aid to produce melt-in-mouth pellets obviating the need of a traditional extrusion-spheronisation aid, MCC. Dual properties of Crospovidone were explored viz. as an extrusion-spheronisation aid and a disintegrant.
Hartauer KJ, Arbuthnot GN, Baertschi SW, Johnson RA, Luke WD, Pearson NG, Rickard EC, Tingle CA, Tsang PK, Wiens RE. Influence of peroxide impurities in povidone and crospovidone on the stability of raloxifene hydrochloride in tablets: identification and control of an oxidative degradation product. Pharm Dev Technol. 2000;5(3):303-10. doi: 10.1081/pdt-100100545.
Abstract. The purpose of this study was to identify a degradation product in a tablet formulation of raloxifene hydrochloride (R-HCl), delineate the role of excipients in its formation, and develop a rational strategy for its control. The degradant was identified as an N-oxide derivative of the drug substance based upon spectroscopic characterization and chromatographic comparison to the synthetic N-oxide. To identify the factors contributing to the formation of N-oxide, binary mixtures of each excipient with R-HCl were exposed to 125 degrees C in open containers. Raloxifene hydrochloride underwent an order of magnitude increase in conversion to the N-oxide in the presence of two excipients, povidone and crospovidone, as compared with its conversion in the presence of other excipients. To confirm a hypothesis that peroxide impurities in these two excipients contributed to the oxidation of the drug substance, tablet lots were spiked with quantities of H2O2 equivalent to 200, 400, 600, and 800 ppm peroxide over the intrinsic levels present in povidone and crospovidone. A strong correlation was observed between the total peroxide level and the quantity of the N-oxide formed upon accelerated storage. From these experiments a rational limit test for peroxide content in povidone and crospovidone was adopted as part of a control strategy to limit formation of the degradation product.
Verheyen P, Steffens KJ, Kleinebudde P. Use of crospovidone as pelletization aid as alternative to microcrystalline cellulose: effects on pellet properties. Drug Dev Ind Pharm. 2009 Nov;35(11):1325-32. doi: 10.3109/03639040902902401.
Abstract. Background: Microcrystalline cellulose (MCC) is the most important pelletization aid in extrusion/spheronization. Because of known disadvantages, the search for substitutes is ongoing. In this context, crospovidone has proven to offer substantial advantages as pelletization aid because of its ability to turn low-soluble active ingredients into fast-dissolving stable pellets. Method: Pellets from crospovidone with different amounts of paracetamol, hydrochlorothiazide, and spironolactone as model drugs were prepared by extrusion/spheronization. For comparison, pellets with MCC as extrusion aid were also produced. The pellets of different formulations were evaluated in terms of yield, aspect ratio, mean Feret diameter, 10% interval fraction, tensile strength, disintegration, and drug release profile. Results: Only crospovidone types exhibiting small particle sizes are suitable as pelletization aid. While maintaining the pharmaceutical quality aspects, it was possible to incorporate up to 60% (w/w) active pharmaceutical ingredients (API) into pellets with crospovidone. The most distinguished differences between pellets based on crospovidone and MCC are the disintegration and drug release behavior. The pellets containing binary mixtures of the low-soluble APIs and crospovidone resulted in fast release in contrast to the pellets with MCC as pelletization aid, which exhibited a slow release. Conclusion: Crospovidone shows an excellent behavior as pelletization aid and produces fast-releasing pellets even with low-soluble APIs.
Shu T, Suzuki H, Hironaka K, Ito K. Studies of rapidly disintegrating tablets in the oral cavity using co-ground mixtures of mannitol with crospovidone. Chem Pharm Bull (Tokyo). 2002 Feb;50(2):193-8. doi: 10.1248/cpb.50.193.
Abstract. We attempted the development of rapid oral disintegration tablets by direct compression using co-ground mixture of D-mannitol and crospovidone. The co-ground mixture was prepared with a vibration rod mill. The tablets were formed by compression using a single punch-tableting machine after addition of the co-ground mixture to non-ground D-mannitol, crospovidone and magnesium stearate. Regarding the properties of tablets, hardness and the time of disintegration were measured. The particle diameter and specific surface area of the co-ground mixture were measured. The tablets manufactured from a physical mixture of 30% (w/w) co-ground mixture of D-mannitol and crospovidone (mixed ratio 9 :1) with 65.5% (w/w) of non-ground mannitol, 4% (w/w) of crospovidone, and 0.5% (w/w) of magnesium stearate had good properties for rapidly disintegrating tablets in the oral cavity. They showed the hardness of 4.9 kg and disintegration time of 33 s. We found that adding co-ground mixture of D-mannitol and crospovidone is useful in enhancing hardness of the tablets that could not be achieved by addition of their individually ground mixture. The improvement in the hardness of the tablets was also observed when other saccharides and disintegrants were used. This method was proved to be applicable in the manufacture of tables of ascorbic acid, a water-soluble drug and nifedipine, a slightly water soluble drug; and the dissolution rate of nifedipine from the tablets in water was remarkably improved. The particle sizes of D-mannitol in the co-ground mixture were smaller than that of the individually ground mixture, resulting in a larger specific surface area of the co-ground mixture than that of the individually ground mixture. Therefore, it was presumed that crospovidone acted as a grinding assistant for D-mannitol in the co-grinding process, enhancing the hardness of tablets by increasing the contact area among powder particles.
Hirasawa N, Ishise S, Miyata H, Danjo K. Application of nilvadipine solid dispersion to tablet formulation and manufacturing using crospovidone and methylcellulose as dispersion carriers. Chem Pharm Bull (Tokyo). 2004 Feb;52(2):244-7. doi: 10.1248/cpb.52.244.
Abstract. Nilvadipine (NIL) solid dispersion using crospovidone (Cross-linked-N-vinyl-2-pyrolidone, cl-PVP) and methylcellulose (MC) as carriers was applied to tablet formulation. Several grades of cl-PVP and MC were used, and their influence on tablet properties such as hardness, disintegration, dissolution and chemical stability were investigated. The agitation granulation method was used for preparation of solid dispersion granules, and the granules were compressed using a rotary tableting machine, and finally the obtained tablets were coated with film. As the particle size of cl-PVP decreased, hardness and apparent solubility were increased, while dissolution rate was lowered. When a higher viscosity grade of MC was used, hardness and dissolution rate were increased, and apparent solubility did not change. All batches of tablets were chemically stable at 40 degrees C, 75% relative humidity (R.H.) for six months. Finally, tablets with enhanced dissolution properties were obtained by using Polyplasdone XL-10 and Metolose SM-25 as the grades of cl-PVP and MC, respectively. These formulation tablets showed higher solubility and dissolution rate during storage as well as initial indicating good physical stability.
Hoyt BS, Aaron DM, Yan S, Linos KD. Cutaneous Crospovidone: A Newly Described Foreign Body Due to Illicit Drug Abuse. Am J Dermatopathol. 2019 Aug;41(8):e84-e86. doi: 10.1097/DAD.0000000000001374.
Abstract. Crospovidone, a polymer of poly N-vinyl-2-pyrrolidone, is an inert insoluble disintegrant found in pharmaceutical tablets. This material has been encountered in the lungs of intravenous drug users and embolized with other components such as talc and microcrystalline cellulose. More recently, crospovidone has also been described in the gastrointestinal tract. We present 2 cases of cutaneous crospovidone deposition resulting from subcutaneous injection of crushed tablets, commonly known as "skin popping." Clinical presentation includes painful, inflamed papules, nodules, or ulcers with overlying eschar. Crospovidone has a distinct and reproducible histochemical staining profile. Histologic recognition of this material is important because it can guide clinicians in their diagnosis and management decisions.
Shaddy SM, Arnold MA, Shilo K, Frankel WL, Harzman AE, Stanich PP, Singhi AD, Yearsley MM, Arnold CA. Crospovidone and Microcrystalline Cellulose: A Novel Description of Pharmaceutical Fillers in the Gastrointestinal Tract. Am J Surg Pathol. 2017 Apr;41(4):564-569. doi: 10.1097/PAS.0000000000000790.
Abstract. Crospovidone and microcrystalline cellulose (MCC) are pharmaceutical fillers well known in the pulmonary pathology literature. Fillers are inactive substances incorporated into medications to facilitate drug delivery. By examining 545 consecutive gastrointestinal surgical specimens from 302 patients between September 11, 2015 and October 23, 2015, we identified the fillers in 29 specimens from 26 patients. The control group consisted of an equal number of consecutive site-matched specimens collected during this same time. Pertinent clinicopathologic data were analyzed, and 1 case was subject to special stains. To confirm the histologic diagnosis, a variety of fillers and medications common to the patients were processed. The fillers were found in 9% of all patients, and there were no specific clinicopathologic associations. In the gastrointestinal tract, crospovidone is nonbirefringent and has a coral shape with each segment composed of a pink core and purple coat; MCC is brightly birefringent with matchstick shape and clear color. Identical material was seen in the processed crospovidone and MCC powders, as well as oxycodone-acetaminophen and omeprazole tablets. In summary, crospovidone and MCC are common, biologically inert, and they are most often seen in the small bowel. Their presence outside of the luminal bowel may serve as a surrogate marker for perforation. Awareness of their morphology is important to distinguish fillers from parasites, calcifications, and other medications, particularly those linked to mucosal injury. We report the unique histomorphologic profile of these fillers as a helpful diagnostic aide, and caution that the fillers have slightly divergent features when compared with those described in the lung.
Shibata Y, Fujii M, Okada H, Noda S, Kondoh M, Watanabe Y. Evaluation of the compaction properties of a solid dispersion of indomethacin with crospovidone by tableting process analyzer. Chem Pharm Bull (Tokyo). 2005 Jul;53(7):759-63. doi: 10.1248/cpb.53.759.
Abstract. A powder solid dispersion system (SD) of indomethacin (IM) with crospovidone (CrosPVP) possesses good fluidity and can be used for tablet formulation. Tablets of SD can be prepared by direct compression and have adequate hardness and a small variation in weight. Forces during the tableting process were measured with a tableting process analyzer (TabAll) equipped with a single-punch. The pressure transmission ratio (PTR) from the upper to the lower punch and the die wall force (DWF) were examined during the tableting process. Ejection force (EF) and scraper pressure (SP) were measured for determining the capping and sticking properties during the tableting process. Adding 1% magnesium stearate (MS) to the SD resulted in high PTR and DWF values and a low EF value. PTR and DWF values increased and EF value decreased when MS and microcrystalline cellulose (MCC) were added to the SD. A thousand tablets could be manufactured without problems such as sticking or capping when 1% MS and 50% MCC were added to the SD containing 25% IM.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Crospovidone Review Consensus 18 by Al222 (20707 pt) | 2024-Oct-08 17:05 |
| Read the full Tiiip | (Send your comment) |
Crospovidone (Povidone K30) or cross-linked polyvinyl N-pyrrolidone or PVP also known as polyvinylpyrrolidone, is a synthetic polymer widely used in pharmaceuticals, cosmetics, and personal care products. It acts as a binder, film-former, stabilizer, and thickening agent in various formulations. Crospovidone is especially valued for its ability to improve the consistency and texture of products, enhance the adhesion of active ingredients to the skin or hair, and stabilize emulsions. In pharmaceuticals, it is commonly used as a binder in tablet formulations and as a stabilizer in liquid medications.
Chemical Composition and Structure
Crospovidone is a water-soluble polymer made from vinylpyrrolidone monomers. The "K30" in its name refers to its molecular weight, indicating the viscosity of the solution it forms. The polymer structure of povidone allows it to form films, bind components, and stabilize formulations. Its excellent solubility in water makes it versatile for use in various aqueous and non-aqueous systems.
Physical Properties
Crospovidone typically appears as a white or off-white powder or granules, easily dissolvable in water and ethanol. It forms a clear, viscous solution upon dissolution and acts as a good film-former and stabilizer. It is non-toxic, non-irritating, and compatible with a wide range of ingredients, making it ideal for use in cosmetics, personal care, and pharmaceutical products.

The name describes the structure of the molecule
- Povidone - Another name for polyvinylpyrrolidone.
- K30 - Indicates the molecular weight grade of the polymer.
Description of raw materials used in production
- Vinylpyrrolidone monomer (N-vinyl-2-pyrrolidone) - The primary raw material for the production of PVP.
Synthesis process
- Radical polymerization - The vinylpyrrolidone monomer undergoes radical polymerization, often using peroxides as the initiator, to form the PVP polymer of varying chain lengths.
- Isolation and purification - The resulting polymer is then isolated, purified, and dried to yield the desired grade of povidone, in this case, K30.
The PVP K series includes: K12, K15, K17, K25, K30, K60, K90.
Applications
- Tablet Binder - Used in pharmaceutical products as a binder in tablets to enhance tablet compression and disintegration.
- Solubilizing Agent - Increases the solubility of poorly water-soluble compounds, thus improving their bioavailability.
- Film-forming Agents - In cosmetic products, Povidone K30 is often used as a film-forming agent, for instance, in hair styling products like gels or sprays.
- Suspension Stabilizer - Can stabilize suspensions in pharmaceutical or cosmetic preparations.
- Viscosifying Agent - Used to increase the viscosity of aqueous solutions or other vehicles.
Crospovidone type A (and type B) is a cross polymer consisting of N-vinylpyrrolidone, often referred to as insoluble PVP or cross-linked PVP. Insoluble in water, acid, alkalis and common organic solvents . It is a light yellow or white hygroscopic powder, practically odourless. Insoluble in water, acid and alkali and in all other common solvents.

Both Crospovidone type A and Crospovidone type B have the characteristic of swelling and leading to the formation of coordination compounds such as polyphenols, carboxylic acid and other low molecular weight compounds. The difference between the two lies in the different density, which leads to sometimes different applications. The higher the density, the finer the powder.
| Crospovidone type A | Density | 1.316 ~ 1.321 g/ml |
| Crospovidone type B | Density | 1.225 ~ 1.342 g/ml |
Tablet compactability. Fine crospovidone grades (type B) increase the tensile strength of tablets at a given compressive pressure. The finer the crospovidone grade, the stronger the bonding capacity.
Tablet compression. Both type A and type B have similar compression values.
Tablet bonding. Copovidone, as a dry binder, increasing the strength of the tablet slows its disintegration. In this case Crospovidone type B can provide rapid disintegration.
What it is used for and where
Medical
By the pharmaceutical industry due to its characteristics of physiological inertness, excellent solubility, film-forming capacity, chemical stability, and biological compatibility, it is used as a medical slow-release carrier, disintegration agent, haemodialysis film, etc. Depending on the ingredients in the formula, the disintegration time of capsules and tablets changes.
The low molecular weight PVP K series serves as a solubilising agent, suspension stabiliser and crystallisation inhibitor in ophthalmic and injectable formulations.
It acts in different ways:
- Disintegrating in solid tablets due to its ability to absorb water and swelling. Crospovidone is inert and has the same chemical structure as povidone, which is used as a carrier in solid dispersion systems. It improves dissolution and bioavailability.
- Complexing agent for tannin and polyphenols in alcoholic extracts or aqueous herbs.
- Suspension stabiliser increases the dispersion capacity of the solution sediment by acting as a hydrophilic viscosising polymer.
- Absorbs moisture
Crospovidone type A is also incorporated into medicinal tablets as a normal binding agent.
Crospovidone type B increases the tensile strength of tablets at a given value of compressive pressure, and the finer the grade of crospovidone, the greater the binding capacity.
Food
In the beverage industry, beer, fruit juices etc., Crospovidone type A has the property of maintaining taste, removing anthocyanin and polyphenol, improving colour and clarity of the liquid. it also acts as a preservative and stabiliser.
Cosmetics
Crospovidone type A acts as a moisturiser for the skin, maintains the moisture of higher grade cosmetics. In toothpastes it acts as a surfactant and alleviates inflammation.
INCI Functions:
- Antistatic agent. Static electricity build-up has a direct influence on products and causes electrostatic adsorption. The antistatic ingredient reduces static build-up and surface resistivity on the surface of the skin and hair.
- Binder agent. Ingredient that is used in cosmetic, food and pharmaceutical products as an anti-caking agent with the function of making the product in which it is incorporated silky, compact and homogenous. The binder, either natural such as mucilage, gums and starches or chemical, may be in the form of a powder or liquid.
- Emulsion stabiliser. Emulsions are thermodynamically unstable. Emulsion stabilisers improve the formation and stability of single and double emulsions. as well as their shelf-life. It should be noted that in the structure-function relationship, the molar mass of the ingredient used plays an important role.
- Film-forming agent. It produces, upon application, a very thin continuous film with an optimal balance of cohesion, adhesion and stickiness on skin, hair or nails to counteract or limit damage from external phenomena such as chemicals, UV rays and pollution.
- Hair fixative. This ingredient has the ability to create, with its protective film, stiffness and hold in the hair, and also has the ability to form, with its hydrophilic and elastic properties, bonds between the hair fibres, to keep the hair in a particular shape for a certain time. In short, it allows physical control of the hairstyle.
- Viscosity control agent. It controls and adapts, Increasing or decreasing, viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
Health and Safety Considerations
Safety in Use
Crospovidone is generally regarded as safe for use in pharmaceutical and cosmetic products. It is non-toxic, non-irritating, and hypoallergenic, making it suitable for a wide range of applications. It is commonly used in oral, topical, and injectable formulations, demonstrating a high level of safety across different use cases.
Allergic Reactions
Allergic reactions to Crospovidone are rare, as it is considered a non-irritating and hypoallergenic ingredient. However, individuals with particularly sensitive skin are advised to perform a patch test before using products containing Crospovidone.
Toxicity and Carcinogenicity
It has been extensively studied and is considered safe at the concentrations used in pharmaceutical, cosmetic, and food products. It is also well-tolerated when administered orally, topically, or intravenously.
Environmental and Safety Considerations
Crospovidone is considered environmentally safe, as it is easily degradable under normal environmental conditions. However, as with all synthetic polymers, it is important to dispose of products containing Crospovidone responsibly to minimize environmental impact.
Regulatory Status
Crospovidone is approved for use in cosmetics, pharmaceuticals, and food products by regulatory agencies such as the European Union and the Food and Drug Administration (FDA) in the United States. It is regulated to ensure that it is used in safe and appropriate concentrations across different products.
The most relevant studies on the subject have been selected with a summary of their contents:
| Appearance | White powder |
| pH | 5-7 |
| Boiling Point | 217.6±7.0°C at 760 mmHg |
| Melting Point | ~165 °C |
| Flash Point | 93.9±0.0°C |
| Density | 1.1±0.1 g/cm3 |
| PSA | 20.31000 |
| LogP | 0.37 |
| Vapor Pressure | 0.1±0.4 mmHg at 25°C |
| Refraction Index | 1.593 |
| Soluble components | ≤1.0% |
| Adsorption | >55 g/100g |
| Loss on drying | ≤5.0 % |
| Soluble components | ≤1.0% |
| Heavy metals | ≤10 ppm |
| Nitrogen | 11.0-12.8% |
| Sulfate ash | ≤0.1% |
| Peroxides H2O2 | ≤400 ppm |
| Aerobic plate count | ≤100 cfu/g |
 |  |
 |  |
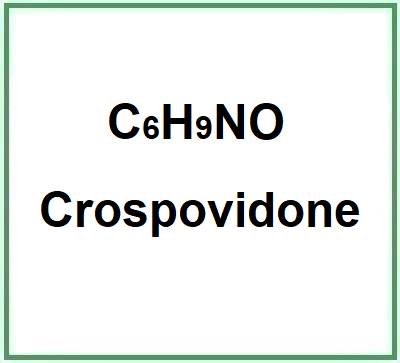
- Molecular Formula C6H9NO C6H13NOP2
- Linear Formula (C6H9NO)n
- Molecular Weight 111.14
- Exact Mass 111.068413
- CAS 25249-54-1 1-Vinyl-2-pyrrolidinone homopolymer (9003-39-8)
- UNII 4LFS24GD0V
- EC Number 201-800-4 607-660-4
- DSSTox Substance ID
- IUPAC 1-Ethenyl-2-pyrrolidinone homopolymer
- InChl=1S/C6H13NOP2/c8-5-2-1-3-7(5)6(10)4-9/h6H,1-4,9-10H2
- InChl Key LQIAZOCLNBBZQK-UHFFFAOYSA-N
- SMILES C1CC(=O)N(C1)C(CP)P
- MDL number MFCD00149016
- PubChem Substance ID
- ChEBI 185135
- RTECS TR8370000
- NCI C80893
- RXCUI 1310706
Synonyms:
- Vinylpyrrolidone
- Polyvinylpyrrolidone
- PVP
- Povidone
- 1-Vinyl-2-pyrrolidinone
- polyvinylpolypyrrolidone
Polyplasdone ; bolinan; colidon; dulcilarmes; hemodyn; kollidon; kollidon 12; kollidon 15; kollidon 17; kollidon 25; kollidon 30; kollidon 40; kollidon 90; kollidon cl; kollidon k30; kollidon k60; kollidon k90; luviskol k30; periston; periston n; plasdone; plasdone 25; plasdone 26 28; plasdone k 29-32; plasdone k 90; plasmosan; poly(n vinyl 2 pyrrolidone); poly(n vinylpyrrolidone); polyvidone; polyvidonk25; polyvinyl polypyrrolidone; poly(vinylpyrrolidone); polyvinylpyrrolidone; polyvinylpyrrolidone; polyvinylpyrrolidone 10 000; polyvinylpyrrolidone 11000; polyvinylpyrrolidone 25000; polyvinylpyrrolidone40000; polyvinylpyrrolidone 44 000; polyvinylpyrrolidone 700 000; polyvinylpyrrolidone solution; poridone; povidone 40; pvp 40000; pvp macrose; rp 143; subtosan; vinisil; 1 vinyl 2 pyrrolidinone polymer; kollidon k 30; polyvidon k25; polyvinyl pyrrolidone; polyvinylpyrrolidone 40000
References__________________________________________________________________________
Liu, Zhao-xia, Qi-lei CHENG, and Lan HE. Impact of the excipient povidone K30 on drug quality control of valsartan capsules. Chinese Journal of Pharmaceutical Analysis 34.6 (2014): 1091-1099.
Abstract. Objective: To confirm the origin and structure of the unknown substance,eluted at 0.14 fold of the relative retention time of valsartan peak (RRT=0.14),which was detected in related substances test of valsartan capsules;to explore the influence of the unknown material on the quality of valsartan capsules,and to provide basis for the revision of the quality standards. Methods: The analysis was carried out on an Inertsil ODS-4 column(250 mm×4.6 mm,5μm)with a mobile phase of acetonitrile-water-glacial acetic acid(500: 500: 1)at a flow rate of 1 mL·min-1.The detective wavelength was set at 225 nm.UPLC-MS,IR,1H-NMR and MALDI-TOF-MS techniques were applied to confirm the origin and structure of the unknown substance. Results: The unknown substance was confirmed to be the excipient povidone K30(PVP K30). Conclusion: The excipient PVP K30 peak is not to be counted as the related substance of valsartan capsules,and it should be specified to deduct the excipient peak in ChP 2010 to eliminate the influence on the result of determination.
Phadke C, Sharma J, Sharma K, Bansal AK. Effect of Variability of Physical Properties of Povidone K30 on Crystallization and Drug-Polymer Miscibility of Celecoxib-Povidone K30 Amorphous Solid Dispersions. Mol Pharm. 2019 Oct 7;16(10):4139-4148. doi: 10.1021/acs.molpharmaceut.9b00452.
Abstract. In the present study, we have investigated the variability in physical properties of povidone K30 (PVP K30) and its impact on crystallization and drug-polymer miscibility of celecoxib-PVP K30 (CLB-PVP K30) amorphous solid dispersions (ASDs). CLB-PVP K30 ASDs were prepared using nine batches of PVP K30, in situ on glass slides by quench-cooling using the hot and cold stage of a microscope. Crystallization of the ASDs stored at 40 ± 2 °C/75 ± 5% relative humidity was captured using polarized light microscopy for up to 24 h and quantified using mean pixel counts of images. The quantitative drug-polymer miscibility of nine CLB-PVP K30 systems was determined using melting point depression. Pearson's correlation analysis was used to find the correlation between (i) % crystallization with drug-polymer miscibility and physical properties and (ii) drug-polymer miscibility and physical properties, of PVP K30. The % crystallization was significantly variable (p < 0.05) among the nine CLB-PVP K30 ASDs. The nine PVP K30 batches exhibited significant variability (p < 0.05) from batch to batch and/or source to source in physical properties. The % crystallization showed correlation to particle size distribution (PSD) (weak positive), glass transition (Tg) (weak positive), drug-polymer miscibility (moderate negative), true density, and porosity (moderate positive) and hygroscopicity (strong positive). Miscibility showed correlation between Tg (weak positive), hygroscopicity (weak negative), PSD (moderate negative), and true density and porosity (strong negative). The study suggests PSD, hygroscopicity, true density, and porosity of PVP K30 as the functionality related characteristics for its intended functionality of physical stability when it is used as a stabilizer in ASDs.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-11-22 10:53:19 | Chemical Risk: |