What is Coumarin
Coumarin is a chemical compound (benzopyran-2-one or chromen-2-one), an aromatic phenylpropanoid belonging to the chemical class of benzopyranes, a colorless crystalline substance in its standard state. The molecule can be described as a benzene molecule fused to an alpha-pyrone ring.
The synthesis process takes place in different steps:
- Preparation. Salicylaldehyde can be synthesized by the oxidation of salicyl alcohol.
- Perkin reaction. Salicylaldehyde enters into a reaction with acetic anhydride in the presence of sodium acetate. This reaction, known as the Perkin reaction, forms cinnamic acid.
- Cyclization. Cinnamic acid is cyclized using phosphorus pentachloride (PCl5) or thionyl chloride (SOCl2), to form coumarin.
It occurs as a fine, white, odourless or crystalline powder.

Where is it ?
It was discovered in Odorata Dipteryx, a tropical fruit-bearing tree, but is present in many plants, e.g. clover. Coumarins, organic heterocycles, are a large family of secondary metabolites found in the plant kingdom. They have a low molecular weight, good bioavailability, high solubility and a simple structure.
What is it for?
Coumarin displays diverse pharmacological properties. It has attracted the attention of chemists and medicinal chemists for decades. Many molecules based on the coumarin ring system have been described utilizing innovative synthetic methods. These synthetic routes have led to interesting analogues of coumarins which possess pharmacological activities like anti-HIV, antimicrobial, antiinflammatory, anticancer, anti-TB, anticonvulsant and inhibitory properties of monoamine oxidases. Details of these studies, correlating structure with biological activity are described in this review (1).
Litsea angulata is a plant species belonging to Lauraceae family that is distributed throughout Indonesia, Malaysia, and New Guinea. The seeds have been traditionally used by local people in Kalimantan, Indonesia for the treatment of boils; however, there is no information about the potency of its branch, bark and leaves yet. This study aimed to determine the antioxidant, antimicrobial activity as well as the phytochemical constituent of Litsea angulata branch, bark, and leaves. L. angulata contains secondary metabolites such as alkaloids, flavonoids, tannins, terpenoids, carotenoids, and coumarin. The antioxidant activity on different plant extracts was a range as very strong to weak capacity. All extracts in this study could inhibit the growth of S. aureus and S. mutans (2).
Used in the electroplating industry and in zinc, cadmium and nickel plating, it can reduce the porosity of the coating and increase the brightness of the coating.
Cosmetics
It is a restricted ingredient as III/77 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009.
Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.
In the cosmetics and food industry is an antimicrobial flavouring and serves to impart taste or fragrance.
It can be chemically produced, but it can also be found in its natural state. No manufacturer specifies it on the label.
Safety in food and cosmetics
In 2004, the European Food Safety Authority ruled out the hypothesis that coumarin produces a genotoxic mechanism, but a tolerable daily intake (TDI) of 0.1 mg/kg body weight was established, however hepatotoxicity in humans cannot be completely excluded for sensitive individuals. Coumarin is known to penetrate easily through the skin and the European Cosmetics Regulation (EU) No 1223/2009 has not set a maximum limit in cosmetics for coumarin. This study considers that, considering an average daily consumption of several cosmetic products, the Tolerable Daily Intake (TDI) can easily be reached and exceeded (3).
It is a product that could give allergy and therefore must be indicated on the label.
The most relevant studies on the subject have been selected with a summary of their contents:
Coumarin studies
Optimal typical characteristics of the commercial product Coumarin
| Appearance | Powder white crystalline |
| Melting point | 69.0℃ ~ 71.0℃ |
| Boiling Point | 298.0±0.0 °C at 760 mmHg |
| Flash Point | 118.3±16.1 °C |
| Density | 1.2±0.1 g/cm3 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.595 |
| PSA | 30.21000 |
| LogP | 1.39 |
| Water Solubility | 1.7 g/L (20 ºC) 1gram clearly dissolved in 8mlv 90% ethanol |
Loss on drying
| ≤2.0% |
Heavy metals
| ≤10 ppm |
| Water | ≤1.0% |
Sulphated ash
| ≤0.5% determined on 1.0 g. |
Residue on ignition
| ≤0.1% |
| Safety |  |
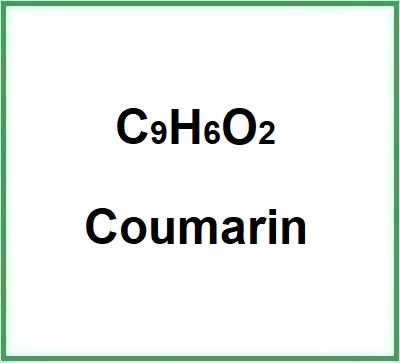
- Formula molecolare : C9H6O2
- Peso molecolare : 146.145 g/mol
- CAS : 91-64-5
- UNII A4VZ22K1WT
- EC Number: 202-086-7
- DSSTox Substance ID: DTXSID7020348
- MDL number
- PubChem Substance ID
- InChI=1S/C9H6O2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-6H
- InChl Key ZYGHJZDHTFUPRJ-UHFFFAOYSA-N
- SMILES C1=CC=C2C(=C1)C=CC(=O)O2
- IUPAC chromen-2-one
- ChEBI 28794
- ICSC 1105
- NSC 755852 8774
- RTECS GN4200000
- UN 2811
- NCI C397
Synonyms :
- Coumarin
- 2H-Chromen-2-one
- 1-Benzopyran-2-one
- 1,2-Benzopyrone
- EINECS 202-086-7
- 2-Propenoic acid, 3-(2-hydroxyphenyl)-delta-lactone
- EC Number: 202-086-7
- MDL number: MFCD00006850
- PubChem Substance ID 329764209
- o-Coumaric acid lactone
- o-Hydroxyzimtsaure-lacton
- Coumarinic anhydride
- 2H-1-Benzopyran-2-one
References_____________________________________________________________________
(1) Srikrishna D, Godugu C, Dubey PK. A Review on Pharmacological Properties of Coumarins. Mini Rev Med Chem. 2018;18(2):113-141. doi: 10.2174/1389557516666160801094919.
(2) Kuspradini H, Wulandari I, Putri AS, Tiya SY, Kusuma IW. Phytochemical, antioxidant and antimicrobial properties of Litsea angulata extracts. F1000Res. 2018 Nov 22;7:1839. doi: 10.12688/f1000research.16620.2.
(3) Stiefel C, Schubert T, Morlock GE. Bioprofiling of Cosmetics with Focus on Streamlined Coumarin Analysis. ACS Omega. 2017 Aug 31;2(8):5242-5250. doi: 10.1021/acsomega.7b00562.
![]() Coumarin
Coumarin