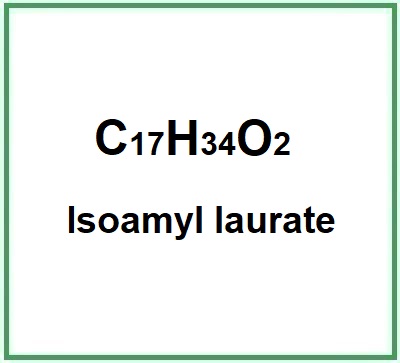
Isoamyl laurate is a chemical compound, a fatty latic acid ester obtained by the condensation of dodecanoic acid with 3-methylbutan-1-ol and derived from an isoamylol. It is industrially produced from vegetable oils and sugar.
The name defines the structure of the molecule:
- "Isoamyl" refers to the isoamyl group, which is a five-carbon alkyl group derived from isopentane. It is often represented as (CH3)2CHCH2-.
- "Laurate" refers to the laurate group, which is a twelve-carbon alkyl group derived from lauric acid. It is often represented as CH3(CH2)10COO-.
The synthesis of isoamyl laurate typically involves the esterification of isoamyl alcohol and lauric acid. Here is a simplified version of the process:
- Isoamyl alcohol is reacted with lauric acid in the presence of an acid catalyst, such as sulphuric acid. This reaction is known as esterification.
- The esterification reaction produces Isoamyl laurate and water. The reaction is reversible, so it is often completed by removing the water that is formed.
The overall process can be summarised as follows:
Isoamyl alcohol + Lauric acid → Isoamyl laurate+ Water
It appears as a colourless transparent liquid. Soluble in ethanol, insoluble in water.

What it is used for and where
Moisturising substitute for silicone oil, of vegetable origin with good compatibility with most epoxy silicone oils, synthetic oils, mineral oils, vegetable oils, silicone oils of various viscosities and sunscreen.
Cosmetics
Used as a solvent, fragrance, as a conditioner in hair conditioners and masks, make-up products for sun protection. Compared to caprylic acid/triglyceride, it has better spreadability, is less sticky and provides a less greasy residual feel on the hands.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Food
Generally used as a flavouring agent in wine, beer.
Typical optimal commercial product characteristics Isoamyl laurate
| Appearance | Colourless transparent liquid |
| Density | 0.856 g/mL at 25 °C(lit.) |
Boiling point
| 170 °C / 2mmHg |
Flash Point
| >230 °F |
| Purity | 99% |
| Water | ≤1.0% |
Index of Refraction
| n20/D 1.436(lit.) |
| PSA | 26.30000 |
| LogP | 5.49660 |
Heavy metals
| ≤10 ppm |
Residue on ignition
| ≤0.1% |
Total Impurities
| ≤0.5% |
Residue on ignition
| ≤0.1% |
| Acidity(mgKOH/g) | 1.0 Max |
| Saponification value (mgKOH/g) | 205.0-215.0 |
- Molecular Formula C17H34O2
- Linear Formula CH3(CH2)10CO2CH2CH2CH(CH3)2
- Molecular Weight 270.5
- Exact Mass 270.25600
- CAS 6309-51-9
- UNII M1SLX00M3M
- EC Number 228-626-1
- DSSTox Substance ID DTXSID3064221
- IUPAC 3-methylbutyl dodecanoate
- InChI=1S/C17H34O2/c1-4-5-6-7-8-9-10-11-12-13-17(18)19-15-14-16(2)3/h16H,4-15H2,1-3H3
- InChl Key FVKRIDSRWFEQME-UHFFFAOYSA-N
- SMILES CCCCCCCCCCCC(=O)OCCC(C)C
- MDL number MFCD00048429
- PubChem Substance ID 24900842
- JECFA 182
- FEMA 2077
- ChEBI 87343
- SCHEMBL 120186
- NSC 42577
- Council of Europe no. 379
Synonyms:
- 3-Methylbutyl dodecanoate
- Isopentyl dodecanoate
![]() Isoamyl laurate
Isoamyl laurate