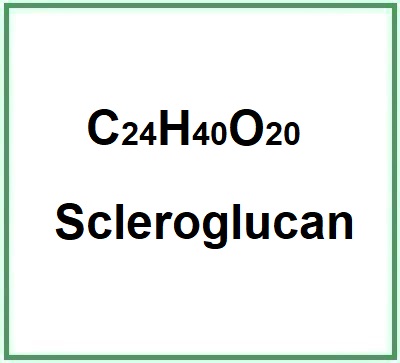
Scleroglucan (β-1,3-β-1,6-glucan) is a typical neutral, non-ionic, high molecular weight, natural microbial β-glucan polysaccharide that is derived non-exclusively from fungi of the genus Sclerotium (Sclerotium rolfsii,S. glucanicume S. delphinii). Compared to synthetic polymers derived from petroleum and natural plant polymers, which have the disadvantage of non-renewable sources, microbial polysaccharides, which have a low production cost and are environmentally sustainable, are the alternative. Scleroglucan is categorised as belonging to the exopolysaccharides which have the advantage of using industrial waste, reduced production times.
Industrially it occurs as a fine white powder with high thermal stability, it is temperature stable up to 100-120°C, soluble in water.

Refined solutions of commercial Scleroglucan have less stable rheological characteristics when exposed to high temperatures, salts, alkalis (1).
What it is used for and where
Scleroglucan has shown anticancer, antiviral and antimicrobial activities and is used in a wide range of industrial and biotechnological applications.
Medical
A number of studies have discussed and demonstrated Scleroglucan's anti-tumour activity (2) and, in the semi-synthetic version used in medicine, its antiviral inhibitory activity capable of blocking a phase of virus replication following virus attachment (3).
Cosmetics
Thickener. Inserted in personal care products to increase viscosity, smoothness and increase the shine and softness of the product (gel or cream) in which it is inserted. Retains moisture in the skin Used in production to decrease the proportional amount of oil in the formula and to prevent pigment separation. Cosmetics manufacturers believe that Scleroglucan has the effect of soothing and smoothing the skin, however, at the moment, we have not found any scientific studies to confirm this claim. Conditioner in hair care products.
Construction
Self-levelling sealants for floor joints and civil engineering applications on concrete with high penetration power.
Oil
Oil recovery, drilling muds.
Scleroglucan studies
Typical optimal commercial product characteristics Scleroglucan
| Appearance | White fine powder |
| Assay | ≥99.0% |
| pH | 6-8 |
| Viscosity(1% water solution) | 600-1200 |
Heavy metal
| ≤10ppm |
| Pb | ≤2ppm |
| Hg | ≤0.05ppm |
| Cd | ≤0.2ppm |
Total bacteria
| Total bacteria |
Yeast and Mould
| ≤20 cfu/g |
Total impurity
| ≤0.5% |
Single impurity
| ≤0.3% |
Residue on lgnition
| ≤0.1% |
Loss on drying
| ≤0.5% |
- Molecular Formula C24H44O20P2 C24H40O20
- Molecular Weight 714.5
- CAS 39464-87-4
- UNII 2X51AD1X3T
- EC Number 254-464-6
- DSSTox Substance ID
- IUPAC 2-[[6-[3,5-dihydroxy-2-(hydroxymethyl)-6-phosphanyloxan-4-yl]oxy-4-[3,5-dihydroxy-6-(hydroxymethyl)-4-phosphanyloxyoxan-2-yl]oxy-3,5-dihydroxyoxan-2-yl]methoxy]-6-(hydroxymethyl)oxane-3,4,5-triol
- InChI=1S/C24H44O20P2/c25-1-5-9(28)13(32)14(33)21(38-5)37-4-8-12(31)18(42-23-16(35)20(44-46)11(30)6(2-26)39-23)15(34)22(40-8)43-19-10(29)7(3-27)41-24(45)17(19)36/h5-36H,1-4,45-46H2
- InChl Key FEBUJFMRSBAMES-UHFFFAOYSA-N
- SMILES C(C1C(C(C(C(O1)OCC2C(C(C(C(O2)OC3C(C(OC(C3O)P)CO)O)O)OC4C(C(C(C(O4)CO)O)OP)O)O)O)O)O)O
- MDL number
- PubChem Substance ID
- ChEBI 184545
Synonyms:
- Polytran
- Sclerotium gum
- Clearogel
- Polytran FS
- Sclerogum
- Sclerosan
- Actigum
- 2-[[6-[3,5-dihydroxy-2-(hydroxymethyl)-6-phosphanyloxan-4-yl]oxy-4-[3,5-dihydroxy-6-(hydroxymethyl)-4-phosphanyloxyoxan-2-yl]oxy-3,5-dihydroxyoxan-2-yl]methoxy]-6-(hydroxymethyl)oxane-3,4,5-triol
- Betasizofiran
References_________________________________________________________________
(1) Wang Y, McNeil B. Scleroglucan. Crit Rev Biotechnol. 1996;16(3):185-215. DOI: 10.3109/07388559609147421.
(2) Singh PP, Whistler RL, Tokuzen R, Nakahara W. Scleroglucan, an antitumor polysaccharide from Sclerotium glucanicum. Carbohydr Res. 1974 Oct;37(1):245-7. doi: 10.1016/s0008-6215(00)87078-0.
Jong SC, Donovick R. Antitumor and antiviral substances from fungi. Adv Appl Microbiol. 1989;34:183-262. doi: 10.1016/s0065-2164(08)70319-8.
(3) Mastromarino P, Petruzziello R, Macchia S, Rieti S, Nicoletti R, Orsi N. Antiviral activity of natural and semisynthetic polysaccharides on the early steps of rubella virus infection. J Antimicrob Chemother. 1997 Mar;39(3):339-45. doi: 10.1093/jac/39.3.339.
(4) Schmid, J., Meyer, V. & Sieber, V. Scleroglucan: biosynthesis, production and application of a versatile hydrocolloid. Appl Microbiol Biotechnol 91, 937–947 (2011). https://doi.org/10.1007/s00253-011-3438-5
![]() Scleroglucan
Scleroglucan