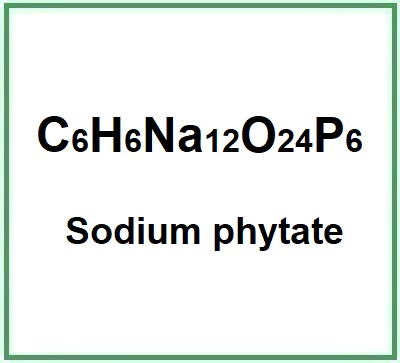
Sodium phytate (Sodium phytate, dodecasodium;(2,3,4,5,6-pentaphosphonatooxycyclohexyl) phosphate) is the salt of phytic acid, a polyphosphate, organic cyclic hexa-phosphate and is responsible in nature for storing phosphorus in grains and seeds.
The name defines the structure of the molecule:
- Sodium (Na). Sodium is an alkaline metal, a chemical element with the symbol Na (from the Latin "Natrium") and the atomic number 11. Sodium is soft and silvery-white in color. It is highly reactive, especially with water, and is commonly found in salts and minerals.
- Phytate refers to the saline form of phytic acid, a substance found in many plant tissues, especially grains and seeds. Phytic acid has the ability to bind to minerals, which can prevent their absorption into the human digestive system.
The synthesis process takes place in several stages:
- Preparation. Phytic acid can be obtained from various plant sources, such as rice bran, wheat bran or soy, plant material is typically soaked in water, and phytic acid is extracted using an acid, such as hydrochloric acid (HCl). The solution is filtered to remove any solid material and phytic acid is precipitated from the solution by adjusting the pH.
- Reaction. Phytic acid is reacted with sodium hydroxide to form sodium phytate. This reaction is carried out in water and the pH of the solution is carefully checked to ensure a complete reaction. The reaction can be represented as follows:
C6H18O24P6 (phytic acid) + 12NaOH (sodium hydroxide) -> Na12C6H18O24P6 (sodium phytate) + 12H2O (water)
- Purification. The crude product of sodium phytate may contain unreacted phytic acid and sodium hydroxide, as well as other impurities, therefore, it is purified to remove these impurities through various methods, such as crystallization, filtration or extraction.
- Characterization.. The final stage of the synthesis process is the characterization of the product to confirm its identity and purity. This can be done through various methods, such as infrared spectroscopy (IR), nuclear magnetic resonance spectroscopy (NMR) and mass spectrometry (MS).
It appears as a white, hygroscopic, water-soluble powder.

What it is used for and where
Food
Sodium phytate has demonstrated ion chelation capabilities together with strong antioxidant activity and, in combination with chitosan, may be useful in extending shelf life by suppressing spoilage in partially frozen food products (1), including in film form using one-step stripping and layer-by-layer-casting technologies (2).
Other studies have examined the effects of sodium phytate on fat digestion and cholesterol metabolism (3).
Industrially it can be added (0.5% ratio) to canned foods to protect their colour. In drinks it can remove excess ions belonging to heavy metals.
Cosmetics
It is a chelating product that helps to keep a cosmetic product stable and, as an antioxidant, has the function of preserving the product from deterioration due to mould, fungus, etc.
Chelating agent. It has the function of preventing unstable reactions and improving the bioavailability of chemical components within a product, and removes calcium and magnesium cations that can cause cloudiness in clear liquids.
Oral care agent. This ingredient can be placed in the oral cavity to improve and/or maintain oral hygiene and health, to prevent or improve a disorder of the teeth, gums, mucous membrane. It provides cosmetic effects to the oral cavity as a protector, cleanser, deodorant.
Other uses
Paints, metallurgy, chemistry.
Sodium phytate studies
Caratteristiche tipiche ottimali del prodotto commerciale Sodium phytate
| Appearance | White powder |
Boiling Point
| 1190.7ºC at 760mmHg |
Flash Point
| 673.9ºC |
| PSA | 493.38000 |
Vapour Pressure
| 0mmHg at 25°C |
| LogP | 2.12580 |
| pH | 8-10 |
| SO42- | ≤ |
| As | ≤ 0.0005% |
| Pb | ≤ 0.0010% |
| Loss on drying | ≤ 10 |
| Cl | ≤ 0.02% |
| Ca | ≤ 0,02% |
- Molecular Formula C6H6Na12O24P6 C6H18O24P6 · xNa+ · yH2O
- Molecular Weight 923.82
- Exact Mass 923.644714
- CAS 14306-25-3
- UNII 3F6PM8J684
- EC Number 238-242-6
- DSSTox Substance ID
- IUPAC dodecasodium;(2,3,4,5,6-pentaphosphonatooxycyclohexyl) phosphate
- InChI=1S/C6H18O24P6.12Na/c7-31(8,9)25-1-2(26-32(10,11)12)4(28-34(16,17)18)6(30-36(22,23)24)5(29-35(19,20)21)3(1)27-33(13,14)15;;;;;;;;;;;;/h1-6H,(H2,7,8,9)(H2,10,11,12)(H2,13,14,15)(H2,16,17,18)(H2,19,20,21)(H2,22,23,24);;;;;;;;;;;;/q;12*+1/p-12
- InChl Key KETSPIPODMGOEJ-UHFFFAOYSA-B
- SMILES C1(C(C(C(C(C1OP(=O)([O-])[O-])OP(=O)([O-])[O-])OP(=O)([O-])[O-])OP(=O)([O-])[O-])OP(=O)([O-])[O-])OP(=O)([O-])[O-].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+]
- MDL number MFCD00082310
- PubChem Substance ID
Synonyms:
- Phytate persodium
- dodecasodium;(2,3,4,5,6-pentaphosphonatooxycyclohexyl) phosphate
References_______________________________________________________________________
(1) Liu B, Pan S. Effect of chitosan coatings incorporated sodium phytate on the shelf-life of Antarctic krill (Euphausia superba). Int J Biol Macromol. 2020 May 15;151:62-65. doi: 10.1016/j.ijbiomac.2020.02.148.
(2) Yang J, Xiong L, Li M, Sun Q. Chitosan-Sodium Phytate Films with a Strong Water Barrier and Antimicrobial Properties Produced via One-Step-Consecutive-Stripping and Layer-by-Layer-Casting Technologies. J Agric Food Chem. 2018 Jun 20;66(24):6104-6115. doi: 10.1021/acs.jafc.8b01890.
(3) Yuangklang C, Wensing T, Lemmens AG, Jittakhot S, Beynen AC. Effect of sodium phytate supplementation on fat digestion and cholesterol metabolism in female rats. J Anim Physiol Anim Nutr (Berl). 2005 Dec;89(11-12):373-8. doi: 10.1111/j.1439-0396.2005.00525.x.
![]() Sodium Phytate
Sodium Phytate