Decylene glycol (1,2-Decanediol) is a chemical compound, alkanediol (composed of alkane and diol), amphiphilic, short-chain 1,2-glycol.
The name describes the structure of the molecule:
- Decylene denotes a chain of ten carbon atoms with a double bond, characteristic of alkenes.
- glycol refers to a molecule with two alcohol groups (-OH), indicating that the substance is a di-alcohol.
Raw Materials Used in Production.
Decylene Glycol is a synthetic diol primarily derived from the chemical reaction between long-chain alkenes and glycolic compounds.
Step-by-step Summary of Industrial Production Process.
- Synthesis of Decylene Glycol through oxidation or hydrogenation of long-chain alkenes.
- Purification of the product to remove impurities and reaction byproducts.
- Quality control to ensure the purity and technical specifications of Decylene Glycol.
It appears as a fine white, water-soluble powder.

What it is used for and where
Medical
In acne, one of the main pathogenic factors is considered to be sebum production and 1,2-Decanediol has been shown in laboratory and in vivo tests to inhibit the biofilm formed by Cutibacterium acnes, a bacterium responsible for acne formation, and to reduce its size (1). 1,2-Decanediol has the potential role of preventing acne vulgaris, without adverse effects (2).
1,2-Decanediol has shown good anti-inflammatory and antioxidant effects (3).
Cosmetics
Alkanediols are used as alternative antimicrobial preservatives for dermal formulations, hydrophilic creams, etc.
Decylene glycol is used in shampoos, liquid soaps as a moisturising agent with antibacterial activity and a thickening and foaming effect.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Chemical
To improve the hydrophilicity and extractability of the compound in multi-residue analysis in supramolecular solvents (SUPRAS) 1,2-decanediol helped to increase the hydrophilic-hydrophobic equilibrium (4).
Commercial Applications
Used in cosmetics and personal care products as a humectant, solvent, and antimicrobial agent. It enhances product stability and shelf life, and contributes to their consistency and applicability.
The most relevant studies on this chemical compound have been selected with a summary of their contents:
1,2-Decanediol studies
| Appearance | White fine powder |
Boiling Point
| 255.0±0.0 °C at 760 mmHg |
Melting Point
| 48°C |
| Density | 0.9±0.1 g/cm3 |
Flash Point
| 122.4±13.0°C 255 ℃/760 mmHg |
| PSA | 40.46000 |
| LogP | 2.38 |
Vapour Pressure
| 0.0±1.1 mmHg at 25°C |
Index of Refraction
| 1.456 |
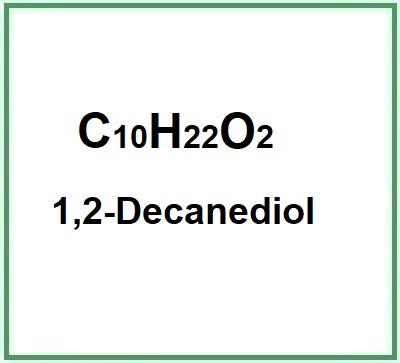
- Molecular Formula C10H22O2
- Linear Formula CH3(CH2)7CH(OH)CH2OH
- Molecular Weight 174.28
- Exact Mass 174.161987
- CAS 1119-86-4
- UNII S57M60MI88
- EC Number 214-288-2
- DSSTox Substance ID
- IUPAC decane-1,2-diol
- InChI=1S/C10H22O2/c1-2-3-4-5-6-7-8-10(12)9-11/h10-12H,2-9H2,1H3
- InChl Key YSRSBDQINUMTIF-UHFFFAOYSA-N
- SMILES CCCCCCCCC(CO)O
- MDL number MFCD00010739
- PubChem Substance ID 24855732
- ChEBI 143858
- SCHEMBL 51461
- NSC 28662
- NACRES NA:23
Synonyms:
- Decane-1,2-diol
- 1,2-DIHYDROXYDECANE
References________________________________________________________________________
(1) Sulzberger M, Fölster H, Sattler M, Rippke F, Grönniger E. Inhibition of Propionibacterium acnes associated biofilm formation by Decanediol. J Dermatol Sci. 2016 Aug;83(2):159-61. doi: 10.1016/j.jdermsci.2016.05.003.
(2) Bassino E, Gasparri F, Munaron L. Pleiotropic Effects of White Willow Bark and 1,2-Decanediol on Human Adult Keratinocytes. Skin Pharmacol Physiol. 2018;31(1):10-18. doi: 10.1159/000481690.
(3) Di Caprio R, Monfrecola G, Balato A, Balato N, Gasparri F, Micillo R, Lembo S. The anti-inflammatory and antioxidant properties of 1,2-decanediol and willow bark extract in lipopolysaccharide-stimulated keratinocytes. G Ital Dermatol Venereol. 2019 Dec;154(6):624-631. doi: 10.23736/S0392-0488.17.05592-4.
(4) González-Rubio S, Ballesteros-Gómez A, García-Gómez D, Rubio S. Double-headed amphiphile-based sponge droplets: synthesis, characterization and potential for the extraction of compounds over a wide polarity range. Talanta. 2022 Mar 1;239:123108. doi: 10.1016/j.talanta.2021.123108.
![]() Decylene glycol
Decylene glycol