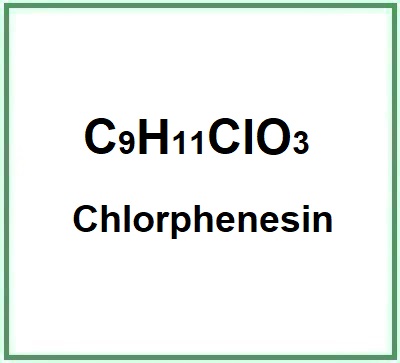
Chlorphenesin (3-p-chlorophenoxy-1,2-propanediol) is a chemical compound, phenolic ether, derived from glycerol and belongs to the class of organic organohalogen compounds. Halogenation is an antibacterial process.
Chemical Industrial Synthesis Process
- Preparation of reagents. The main reagents include p-chlorophenol and glycerin, which are the raw materials needed for the synthesis of Chlorphenesin.
- Esterification reaction. Glycerin and p-chlorophenol are combined in the presence of a dehydrating agent, such as sulfuric acid, to facilitate ester formation.
- Monitoring the reaction. The reaction is closely monitored to ensure it proceeds under optimal temperature and time parameters, thus maximizing the product yield.
- Purification. The crude product is purified through techniques such as distillation or crystallization to remove impurities and enhance the purity of Chlorphenesin.
- Quality control. The purified Chlorphenesin undergoes various chemical and microbiological tests to verify its purity, efficacy, and safety.
It appears in the form of a white powder

A cosa serve e dove si usa
Antibacterial and antifungal agent (active against Gram-positive and Gram-negative bacteria). The maximum allowable dosage is 0.3 percent in rinsing products. (1).
Chlorphenesin is inserted in cosmetic formulations for its effectiveness in protecting products from microbial contamination. This preservative not only extends the product's shelf life but also ensures safety in use by preventing the growth of harmful microorganisms. It is commonly used in a wide range of cosmetic products, including creams, lotions, and skincare products, where it helps maintain the integrity and quality of the product over time.
Cosmetics
It is a restricted cosmetic ingredient as V/50 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: 3-(p-Chlorophenoxy)-propane-1,2-diol. Maximum concentration in ready for use preparation 0.3%
Cosmetics - INCI Functions
- Antimicrobial agent. This ingredient is able to suppress or inhibit the growth and replication of a broad spectrum of microorganisms such as bacteria, fungi and viruses by making the stratum corneum temporarily bactericidal and fungicidal.
- Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Safety
Scientific literature warns about general and specific contraindications involving the application of this preservative. Specifically, with regard to the eyes, this 2020 study by Jingyi Wang and others at Harvard University warns about the toxicity of Chlorphenesin to the epithelial cells of the Meibomian gland, which secretes, through its ducts, the human tear film, a major source of lipid (2).
Chlorphenesin studies
| Appearance | White fine powder |
Boiling Point
| 369.5±27.0 °C at 760 mmHg |
| Melting Point | 77-79°C |
| Density | 1.3±0.1 g/cm3 |
Flash Point
| 177.2±23.7°C |
Index of Refraction
| 1.565 |
Vapour Pressure
| 0.0±0.9 mmHg at 25°C |
| LogP | 1.43 |
| PSA | 49.69000 |
| Safety |  |
- Formula molecolare C9H11ClO3
- Peso molecolare 202.63
- Massa esatta 202.039673
- CAS 104-29-0
- UNII I670DAL4SZ
- EC Number 203-192-6
- DSSTox Substance ID DTXSID0049028
- IUPAC 3-(4-chlorophenoxy)propane-1,2-diol
- InChI=1S/C9H11ClO3/c10-7-1-3-9(4-2-7)13-6-8(12)5-11/h1-4,8,11-12H,5-6H2
- InChl Key MXOAEAUPQDYUQM-UHFFFAOYSA-N
- SMILES C1=CC(=CC=C1OCC(CO)O)Cl
- MDL number
- PubChem Substance ID
- ChEBI 3642
- NSC 6401
- RTECS TY4260000
Synonyms:
- 3-(4-chlorophenoxy)propane-1,2-diol
- p-Chlorophenyl glyceryl ether
References___________________________________________________________________
(1) Johnson W Jr, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Safety Assessment of Chlorphenesin as Used in Cosmetics. Int J Toxicol. 2014 May;33(2 suppl):5S-15S. doi: 10.1177/1091581814526893.
(2) Wang J, Liu Y, Kam WR, Li Y, Sullivan DA. Toxicity of the cosmetic preservatives parabens, phenoxyethanol and chlorphenesin on human meibomian gland epithelial cells. Exp Eye Res. 2020 Jul;196:108057. doi: 10.1016/j.exer.2020.108057.
![]() Chlorphenesin
Chlorphenesin