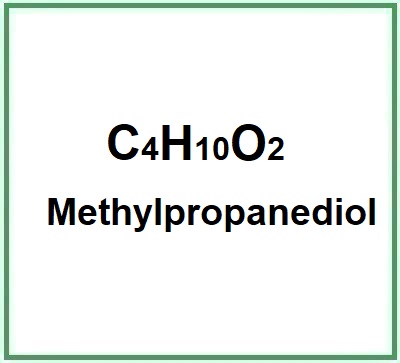
![]() Methylpropanediol
Methylpropanediol
Rating : 7
Methylpropanediol (2-Methyl-1,3-propanediol) is a chemical organic compound, with low molecular weight, a low viscosity liquid glycol.The name describes the structure of the molecule:Methyl. Indicates the presence of a methyl group (-CH3) in the molecule.Propane. A three-carbon chain (propane).Diol. This suffix indicates the presence of two h... (Read the full Tiiip)
10 pts from Ark90
| Evaluate | Where is this found? |
| "Methylpropanediol studies" about Methylpropanediol Review Consensus 9 by Ark90 (12432 pt) | 2022-Mar-08 12:12 |
Compendium of the most significant studies with reference to properties, intake, effects.Bährle-Rapp, M. (2007). Methylpropanediol. In Springer Lexikon Kosmetik und Körperpflege (pp. 353-353 ...
| Read the full Tiiip | (Send your comment) |
| "Descrizione" about Methylpropanediol Review Consensus 10 by Ark90 (12432 pt) | 2023-Dec-01 22:19 |
Methylpropanediol (2-Methyl-1,3-propanediol) is a chemical organic compound, with low molecular weight, a low viscosity liquid glycol.The name describes the structure of the molecule:Methyl. Indicates ...
| Read the full Tiiip | (Send your comment) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-03-08 11:00:47 | Chemical Risk: |