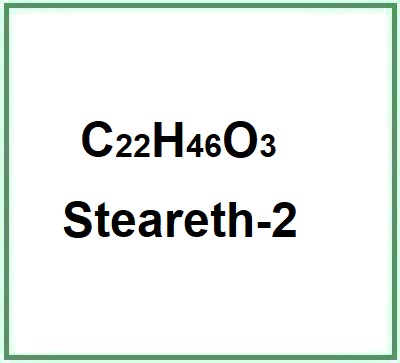
![]() Steareth-2
Steareth-2
Rating : 5
Cons:
Ethoxylated chemical compound (1)Steareth-2 is a chemical compound, carbon chains alkyl polyethylene glycol (PEG (Polyethylene glycol)) ether of ethoxylated fatty stearyl alcohol (2 mol), from palm kernel oils and coconut oils and fats.Safety. The term 'eth' refers to the ethoxylation reaction with ethylene oxide after which residues of ethyle... (Read the full Tiiip)
9 pts from Ark90
| Evaluate | Where is this found? |
| "Steareth-2 studies" about Steareth-2 Review Consensus 8 by Ark90 (12432 pt) | 2022-Mar-09 15:56 |
Compendium of the most significant studies with reference to properties, intake, effects.Bárány E, Lindberg M, Lodén M. Unexpected skin barrier influence from nonionic emulsi ...
| Read the full Tiiip | (Send your comment) |
| "Descrizione" about Steareth-2 Review Consensus 9 by Ark90 (12432 pt) | 2023-Mar-08 14:45 |
Steareth-2 is a chemical compound, carbon chains alkyl polyethylene glycol (PEG (Polyethylene glycol)) ether of ethoxylated fatty stearyl alcohol (2 mol), from palm kernel oil ...
| Read the full Tiiip | (Send your comment) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-03-09 20:10:51 | Chemical Risk: |