![]() Cetearyl glucoside
Cetearyl glucoside
Rating : 7.5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from FRanier
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Cetearyl glucoside studies" about Cetearyl glucoside Review Consensus 10 by FRanier (9971 pt) | 2022-Apr-02 17:15 |
| Read the full Tiiip | (Send your comment) |
Cetearyl glucoside. Compendium of the most significant studies with reference to properties, intake, effects.
Pantelic, Ivana, ed. Alkyl Polyglucosides: From natural-origin surfactants to prospective delivery systems. Woodhead Publishing, 2014.
Abstract. Sugar-based surfactants represent a growing market. Among these, Alkyl Polyglucosides produced from vegetable oil and starch, are in demand.
Djuris J, Vasiljevic D, Jokic S, Ibric S. Application of D-optimal experimental design method to optimize the formulation of O/W cosmetic emulsions. Int J Cosmet Sci. 2014 Feb;36(1):79-87. doi: 10.1111/ics.12099.
Abstract. This study investigates the application of D-optimal mixture experimental design in optimization of O/W cosmetic emulsions.
Savić S, Weber C, Savić MM, Müller-Goymann C. Natural surfactant-based topical vehicles for two model drugs: Influence of different lipophilic excipients on in vitro/in vivo skin performance. Int J Pharm. 2009 Nov 3;381(2):220-30. doi: 10.1016/j.ijpharm.2009.07.007.
Abstract. This study focuses on the properties of topical vehicles based on alkylpolyglucoside natural surfactant-mixed emulsifier, cetearyl glucoside and cetearyl alcohol, in order to propose their use as "ready to use" pharmaceutical bases for a number of model drugs. We were interested to investigate how the alternative use of three lipophilic excipients (Ph. Eur. 6.0), differing in their polarity indexes (medium chain triglycerides (MG), decyl oleate (DO), and isopropyl myristate (IPM), respectively), affects the colloidal structure of the alkylpolyglucoside-based vehicles and in vitro permeation profiles of two model drugs: diclofenac sodium (DC) and caffeine (CF), both sparingly soluble in water. Finally, we aimed to evaluate the safety profile of such vehicles in vitro (acute skin irritation test using a cytotoxicity assay), comparing it with in vivo data obtained by the methods of skin bioengineering. The results have shown that the emulsion vehicles consisted of a complex colloidal structure of lamellar liquid crystalline and lamellar gel crystalline type.
Bhoyrul, Bevin, et al. "Patch testing with alkyl glucosides: Concomitant reactions are common but not ubiquitous." Contact dermatitis 80.5 (2019): 286-290.
Abstract. Alkyl glucosides contitute a family of mild surfactants that are increasingly being used in a wide range of cosmetics and household products. Contact allergy to alkyl glucosides may be more frequent than previously suspected, especially in atopic patients. Objectives. To investigate the frequency of contact allergy to alkyl glucosides, and to identify concomitant reactivity.
Savic S, Weber C, Tamburic S, Savic M, Müller-Goymann C. Topical vehicles based on natural surfactant/fatty alcohols mixed emulsifier: The influence of two polyols on the colloidal structure and in vitro/in vivo skin performance. J Pharm Sci. 2009 Jun;98(6):2073-90. doi: 10.1002/jps.21591.
Abstract. There is a growing need for in-depth research into new skin- and environment-friendly surfactants, such as alkylpolyglucosides. The aim of this study was to assess whether, to which extent and by what mechanism the two commonly used hydrophilic excipients, propylene glycol (PG) and glycerol (GL), affect the colloidal structure of emulsions formed by a natural mixed emulsifier, cetearyl glucoside and cetearyl alcohol.
Montenegro, L., Carbone, C., Paolino, D., Drago, R., Stancampiano, A. H., & Puglisi, G. (2008). In vitro skin permeation of sunscreen agents from O/W emulsions. International journal of cosmetic science, 30(1), 57-65.
Abstract. The effects of different emulsifiers on the in vitro permeation through human skin of two sunscreen agents [octylmethoxycinnamate (OMC) and butylmethoxydibenzoylmethane (BMBM)] were investigated from O/W emulsions.
Savic, S., Vuleta, G., Daniels, R., & Müller-Goymann, C. C. (2005). Colloidal microstructure of binary systems and model creams stabilized with an alkylpolyglucoside non-ionic emulsifier. Colloid and Polymer Science, 283(4), 439-451.
Abstract. The aim of this study was to examine the lyotropic potential of an alkylpolyglucoside mixed emulsifier (Cetearyl glucoside&Cetearyl alcohol), which belongs to the new generation of natural (sugar) surfactants, and to elaborate the potential stabilization mechanism and relation between the colloid microstructure and water distribution within the systems.
Baudy A, Dereure O, Du-Thanh A, Raison-Peyron N. Allergic contact dermatitis in response to cetearyl glucoside from a topical drug. Contact Dermatitis. 2022 Feb;86(2):125-127. doi: 10.1111/cod.13985.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Cetearyl glucoside Review Consensus 10 by FRanier (9971 pt) | 2024-Oct-07 16:37 |
| Read the full Tiiip | (Send your comment) |
Cetearyl glucoside is a chemical compound that is part of the Alkylpolyglucosides ingredients obtained by reacting an alcohol or alcohol mixtures with a cyclic form of natural glucose or sugar. Cetearyl glucoside is also produced industrially in a waxy form by extraction from maize and coconut.
Cetearyl Glucoside is a natural emulsifier derived from plant-based raw materials such as cetearyl alcohol (a blend of fatty alcohols) and glucose. It is widely used in skin and hair care products, where it helps to stabilize emulsions by blending water-based and oil-based ingredients. Thanks to its gentle emollient properties, it improves the texture of products, making them creamier and easier to apply without leaving a greasy residue.
Chemical Composition and Structure
Cetearyl Glucoside is a compound formed from the reaction of cetearyl alcohol (a mixture of cetyl and stearyl alcohol) and glucose. Its chemical structure combines a long fatty acid chain with a sugar molecule, allowing this emulsifier to bind both water and oil molecules, thus stabilizing emulsions.
Physical Properties
Cetearyl Glucoside typically appears as a white or yellowish waxy solid. It is oil-soluble and disperses in water, making it ideal for use in oil-in-water (O/W) or water-in-oil (W/O) emulsions. It provides a lightweight and silky feel on the skin and improves the stability and texture of cosmetic formulations.
Production Process
Cetearyl Glucoside is produced through a synthesis process involving the esterification of cetearyl alcohol and glucose. Both ingredients are plant-based, making this emulsifier a popular choice for natural and organic formulations. It is biodegradable and suitable for use in eco-friendly products.
- Cetearyl denotes the cetearyl group, which is a mixture of fatty alcohols, predominantly cetyl and stearyl alcohols. These are long-chain alcohols, with the cetyl alcohol having 16 carbon atoms and the stearyl alcohol 18 carbon atoms.
- glucoside refers to a glycoside derived from glucose. Glycosides are molecules in which a sugar is bound to a non-glucidic part, usually a small organic molecule. In this case, the glucose is bound to the cetearyl group.
Description of the raw materials used in its production:
- Cetearyl alcohol is a mixture of fatty alcohols, primarily cetyl alcohol and stearyl alcohol. It is derived from natural sources such as coconut or palm oil.
- Glucose is a simple sugar that is commonly derived from corn or other plant sources.
Industrial chemical synthesis step-by-step:
- Esterification - Cetearyl glucoside is synthesized through the reaction of cetearyl alcohol with glucose. This esterification reaction involves the combination of the hydroxyl group of cetearyl alcohol with the glucose molecule, resulting in the formation of cetearyl glucoside.
- Purification - The crude product obtained from the esterification reaction is purified to remove any impurities or by-products. Purification techniques such as filtration, distillation, or recrystallization may be employed.
- Quality Control - The purified cetearyl glucoside undergoes quality control tests to ensure its purity, stability, and compliance with industry standards.
- Formulation - Cetearyl glucoside is commonly used as an emulsifier and stabilizer in various cosmetic and personal care formulations, including lotions, creams, and moisturizers. It helps to blend and stabilize the oil and water phases of these products, resulting in a smooth and uniform texture.
It occurs as a white flaky or granular powder or as a transparent yellowish liquid.

What it is used for and where
Biodegradable non-ionic surfactant
Cosmetics
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
It is used in oil-in-water creams with high oil content, including silicones and vegetable oils, lotions, foundations, sunscreens, cleansing creams, hair care, whitening, anti-wrinkle and other personal care products as an emulsifying agent. It has a high electrolyte tolerance and a wide Ph 3-12 range.
Applications
- Emulsifier - Promotes the formation of stable emulsions, helping to mix oil and water phases in products such as creams and lotions.
- Skin Conditioning Agent - Can make the skin feel soft and smooth, enhancing the overall feel of products on the skin.
- Natural and Organic Products - Given its natural origin, Cetearyl Glucoside is often favored in formulations of natural and organic products.
- Skin Compatibility - It's known to be gentle and well-tolerated, making it suitable for products intended for sensitive skin.
- Hair Care Products - Can be used in shampoos and conditioners to improve their consistency and emulsifying properties.
Health and Safety Considerations
Safety in Use
Cetearyl Glucoside is considered safe for use in cosmetic products. It is well tolerated by most people, including those with sensitive skin. There are no significant side effects or toxicity issues associated with its use in cosmetic formulations.
Allergic Reactions
Allergic reactions to Cetearyl Glucoside are rare. However, as with any cosmetic ingredient, it is advisable to perform a patch test before using products containing it, especially for individuals with particularly sensitive or reactive skin.
Toxicity and Carcinogenicity
There is no evidence that Cetearyl Glucoside is toxic or carcinogenic. It is considered a safe, natural, and biodegradable ingredient with no health risks when used appropriately in cosmetic products.
Environmental and Safety Considerations
Cetearyl Glucoside is biodegradable and derived from renewable sources, making it an eco-friendly and sustainable choice for cosmetic formulations. Its environmental impact is minimal, making it suitable for use in certified organic or eco-friendly products.
Regulatory Status
Cetearyl Glucoside is approved for use in cosmetics in many regions, including the European Union and the United States. It is used in compliance with safety regulations to ensure the efficacy and safety of cosmetic products.
| Appearance | White flake or granules |
| Melting Point | ≤62℃ |
| pH | 4.0~8.0 |
| Water content | ≤2.0% |
| Acid Value | Acid Value |
| Hydroxyl Value | 270~290 |
| Iodine | ≤1.0 |
| Chemical Safety |  |
 |  |
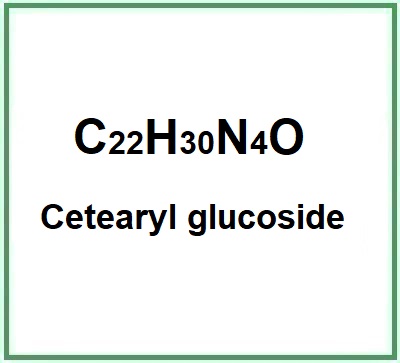
- Molecular Formula C22H30N4O
- EC number 236-131-7
- CAS 246159-33-1
Synonyms:
- C16-18-alkyl glycosides
References_____________________________________________________________________
Bhoyrul B, Solman L, Kirk S, Orton D, Wilkinson M. Patch testing with alkyl glucosides: Concomitant reactions are common but not ubiquitous. Contact Dermatitis. 2019 May;80(5):286-290. doi: 10.1111/cod.13186.
Abstract. Background: Alkyl glucosides contitute a family of mild surfactants that are increasingly being used in a wide range of cosmetics and household products. Contact allergy to alkyl glucosides may be more frequent than previously suspected, especially in atopic patients....Conclusions: The prevalence of alkyl glucoside-induced ACD is relatively high, and there are frequent concomitant reactions between different alkyl glucosides. We recommend the inclusion of alkyl glucosides in all cosmetic series. © 2018 John Wiley & Sons A/S.
Savić S, Savić M, Tamburić S, Vuleta G, Vesić S, Müller-Goymann CC. An alkylpolyglucoside surfactant as a prospective pharmaceutical excipient for topical formulations: the influence of oil polarity on the colloidal structure and hydrocortisone in vitro/in vivo permeation. Eur J Pharm Sci. 2007 Apr;30(5):441-50. doi: 10.1016/j.ejps.2007.01.006.
Abstract. There is a growing need for research into new skin- and environment-friendly surfactants. This paper focuses on a natural surfactant of an alkylpolyglucoside type, which can form both thermotropic and lyotropic liquid-crystalline phases. The aim of this study was to relate some physicochemical properties (characterised by polarisation and transmission electron microscopy, thermal analysis and rheology) of the three formulations based on cetearyl glucoside and cetearyl alcohol, to the results of in vitro and in vivo bioavailability of hydrocortisone (HC). The three formulations contained oils of different polarity (medium chain triglycerides: MG, isopropyl myristate: IPM and light liquid paraffin: LP), respectively. In vitro permeation was followed through the artificial skin constructs (ASC), while the parameters measured in vivo were erythema index: EI (using instrumental human skin blanching assay), transepidermal water loss (TEWL) and stratum corneum hydration (SCH). The vehicles based on cetearyl glucoside and cetearyl alcohol showed a complex colloidal structure of lamellar liquid-crystalline and lamellar gel-crystalline type, depending on oil polarity. Rheological profile of the vehicle was directly related to the in vitro profile of the HC permeation. In vivo results suggested that the vehicle with MG retarded the HC permeation, whereas less polar IPM and non-polar LP enhanced it. It is suggested that the enhancement is achieved either by a direct interaction with lipid lamellae of the SC or indirectly by improving skin hydration. There were no adverse effects during in vivo study, which indicates a good safety profile of this alkylpolyglucoside surfactant.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-04-02 12:25:30 | Chemical Risk: |