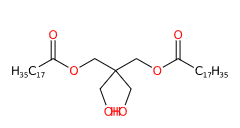
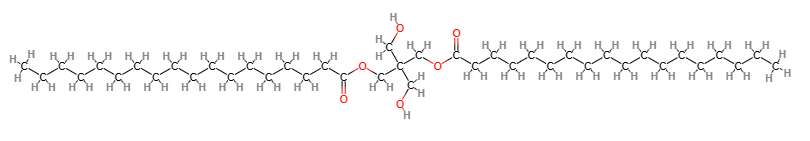
Pentaerythrityl distearate is a chemical compound produced by the esterification of pentaerythritol with stearic acid.
The name defines the structure of the molecule:
- Pentaerythrityl refers to a compound derived from pentaerythritol, a type of alcohol with four hydroxyl groups (-OH). Pentaerythritol is often used in the production of resins and polymers.
- Distearate refers to the diester or saline form of stearic acid, a type of saturated fatty acid commonly found in animal and vegetable fats. The prefix "di-" indicates that there are two Stearati groups in the molecule.
The synthesis process takes place in several stages:
- Acid catalysis. In the first phase the process begins with an acid catalyst to accelerate the reaction, a strong acid such as sulfuric acid (H2SO4).
- Esterification. Pentaerythritol, an alcohol and stearic acid are mixed together in the presence of the acid catalyst that gives a proton to alcohol, making it a better starting group. Carboxylic acid then attacks the protonated alcohol, forming an intermediate.
- Removal of water. As the reaction proceeds, water is formed as a by-product. The reaction is based on balance, so removing water as it forms can help guide the reaction to completion.
- Purification. The resulting mixture is purified with a distillation treatment, to isolate Pentaerythrityl distearate.
- Neutralization. Any residual acid is neutralized, often with a base, to prevent further reactions.
It appears as a fine, white or yellowish crystalline powder.

What it is used for and where
Pentaerythrityl Distearate is a diester formed by pentaerythritol and stearic acid. It is often used in cosmetics and personal care products for its emollient and thickening properties. It can help soften the skin, provide a smooth texture to products and help stabilize emulsions.
Cosmetics
Used as an emulsifying or co-emulsifying agent to increase the viscosity and consistency of a product. Added in lipsticks to improve gloss. As a substitute for beeswax it can increase the lubricity and consistency of a product and reduce dryness. It is included in formulations of shampoos, sunscreen, creams and personal care products.
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Other applications
The mixed mixture of mono and distearate ester is particularly useful in wire and cable compounds to improve yield and provide a smooth surface. Extrusion of rigid PVC profiles.
| Appearance | White power to Light yellow |
| Boiling Point | 680.8°C at 760 mmHg |
Melting Point
| 72°C |
Flash Point
| 183.7ºC |
| Density | 0.945g/cm3 |
| PSA | 93.06000 |
| LogP | 11.56680 |
Vapour Pressure
| 1.82E-21mmHg at 25°C |
| Index of Refraction | 1.473 |
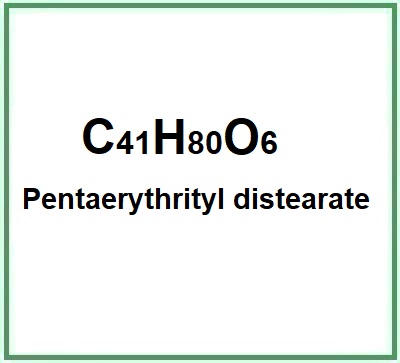
- Molecular Formula C41H80O6
- Molecular Weight 669.1
- Exact Mass 668.59500
- CAS 13081-97-5
- UNII 697WOT8HNB
- EC Number 235-991-0
- DSSTox Substance ID DTXSID4047175
- IUPAC [2,2-bis(hydroxymethyl)-3-octadecanoyloxypropyl] octadecanoate
- InChI=1S/C41H80O6/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-39(44)46-37-41(35-42,36-43)38-47-40(45)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h42-43H,3-38H2,1-2H3
- InChl Key FSEJJKIPRNUIFL-UHFFFAOYSA-N
- SMILES CCCCCCCCCCCCCCCCCC(=O)OCC(CO)(CO)COC(=O)CCCCCCCCCCCCCCCCC
- MDL number MFCD00059225
- PubChem Substance ID 87574885
Synonyms:
- Pentaerythritol Distearate
- Octadecanoic acid,2,2-bis(hydroxymethyl)-1,3-propanediyl ester
- Pentaerythrit-distearat
- 2,2-bis(hydroxymethyl)propane-1,3-diyl distearate
![]() Pentaerythrityl distearate
Pentaerythrityl distearate